Persistent cardiac arrhythmias are readily amenable to detection by performing a standard electrocardiogram (ECG), but detection of transient (paroxysmal) arrhythmias has long been a significant cause of frustration to both doctors and patients. Often a significantly symptomatic arrhythmia is experienced by the patient but terminates before an ECG can be recorded to allow diagnosis. Prognostically important treatment is often delayed, and recurrent symptomatic attacks represent a high morbidity in patients’ lives and result in a burden on emergency services, who often arrive after the arrhythmia has terminated with no resultant progress in making a diagnosis.
Another area of concern has been the presence of asymptomatic, but clinically important, arrhythmias that can go unnoticed by people experiencing them and may result in permanent harm; asymptomatic paroxysmal atrial fibrillation in patients with high CHA2DS2-VASc scores being the most common example.
Both these issues are now being importantly addressed by the widespread availability of portable ECG recording devices, which patients can either manually activate themselves or program to automatically detect abnormal arrhythmias. Information on the range of devices available and their strengths and weaknesses is limited. This article aims to provide a helpful overview for patients and doctors advising them.
Background

Some paroxysmal arrhythmias are either too short in duration, or result in symptoms too severe, to allow patients to be able to activate and record an electrocardiogram (ECG) on a portable patient-activated monitor. Non-sustained ventricular tachycardia, sinus pauses and transient high-grade atrioventricular block can be examples of this. Many paroxysmal arrhythmias, however, have a duration of at least a few minutes during which, a patient familiar with the use of a personal ECG-recording device, can activate the device and record an ECG that is of sufficient quality for a cardiologist to review the recording and determine the diagnosis, and initiate appropriate investigation and treatment.
Equally, the presence of asymptomatic arrhythmias, perhaps even occurring during sleep, can be detected and recorded by some continuously worn devices, such as smart watches. These are particularly important in the detection of atrial fibrillation (AF). Increasingly, smart watches automatically record such episodes when detected.
Personal technologies for arrhythmia detection
There are two main recording technologies: ECG and photoplethysmography (PPG).1-3 In ECG recording devices, non-12-lead ECGs are recorded by electrodes on the device; participants simply place their fingers on the electrodes, attach the electrodes to their wrists or chest, or hold the electrodes in their hands. These devices provide an actual ECG recording, which can subsequently be shared with a clinician.
PPG is a technology that measures changes in tissue blood volume that enables the recording of each heartbeat; the signal can be detected by any device with a camera monitoring various body parts, including the fingertip, wrist, palm, and face. Such recordings can detect rate and irregularity of the heart rhythm but do not provide an ECG recording.
Both non-12-lead ECG and PPG technologies offer high diagnostic accuracies for AF. Today, artificial intelligence (AI) in cardiac devices detects AF with a high accuracy using ECG or PPG. Demonstrating effectiveness, convenience, and time savings with high diagnostic accuracies, these technologies with built-in automatic interpretation can be used as preliminary screening tools for the detection of AF when the gold-standard method, which generally requires a physician’s interpretation, is not feasible. Some, such as the freely available PPG technology Fibricheck system, can be downloaded as an app on to most smartphones without the need for any supplementary device, and are a useful screening tool for AF, but cannot provide an ECG.4
Wearable personal ECG monitors are effective methods of screening for asymptomatic AF. In the 2020 European Society of Cardiology (ESC) and European Association of Cardio-Thoracic Surgery (EACTS) guidelines for the diagnosis and management of AF,5 it states:
- Opportunistic screening for AF is recommended in patients ≥65 years old, hypertensive patients, and in patients with obstructive sleep apnoea. Systematic ECG screening should be considered to detect AF in patients aged ≥75 years, or those at high risk of stroke.
Patients with high CHA2DS2-VASc scores may benefit from wearing personal ECG monitoring devices to detect asymptomatic atrial fibrillation and start early preventative treatment with direct oral anticoagulants (DOACs).
The current UK National Screening Committee (UK NSC) policy, based on its last review in August 2019,6 is that population screening for AF should not be offered by the National Health Service (NHS). The UK NSC’s review of AF screening was due to be completed by 31 March 2023 but has not been updated yet. The UK NSC does not recommend a national screening programme for AF because:
- there are different types of AF and it is not clear if these all have the same risk for stroke
- it is not known how effective treatment for AF is in people found through screening
- it is not known if screening is more beneficial for people with AF than the current approach.
A large research project is under way in the UK to find out if screening is more beneficial for people with AF than the current process. This will help the UK NSC to understand more about the benefits and harms of screening.6 The high rate of asymptomatic AF is well documented across many countries, supporting the argument for screening.7–10
A large number of devices are appearing on the market, but, from our own experience, the most common devices in use at present in the UK seem to be those in table 1. Examples of ECGs recorded by patients using personal ECG recording devices are shown in figure 1.
Table 1. Commonly used smart personal devices
| Type of device | Company | Records ECG | Requires mobile phone + app | Battery life | Size | Other features | Price range |
|---|---|---|---|---|---|---|---|
| Apple Watch 6, 7 or 8 | Apple | Yes | Yes Designed for iOS iPhones |
24-hour rechargeable | 41–45 mm | Yes | £400–500 |
| Samsung Galaxy Watch 4 or 5 | Samsung | Yes | Yes Designed for Android and Samsung phones |
24-hour rechargeable | 40–44 mm | Yes | £200–250 |
| Withings ScanWatch | Withings | Yes | Yes iOS and Android compatible |
12 months/30 days + 20 days | 38–42 mm | Yes – limited | £250–350 |
| Fitbit Charge 5 | Fitbit | Yes | Yes Compatible with iOS ≥13 or Android ≥8.0 |
5 days | 1.04 inch display | Yes | £99–180 |
| AliveCor KardiaMobile and KardiaMobile6L | AliveCor | Yes | Yes, cannot be used without a smartphone | 200 hours | 8.2 × 3.2 × 0.35 cm and 9 × 3 × 0.72 cm |
No | £99–149 |
| EMAY ECG monitor | EMAY | Yes | No, has a basic screen display | 500 readings | 10 × 4.5 × 1.5 cm | No | £90–200 |
| Wellue Pulsebit Ex portable ECG monitor; with OLED screen | Wellue | Yes | No, has a screen and can record without phone | 100 × 30 second ECG storage Readings/strap-free – 48 hours |
3.5” × 2.2” × 0.5” or 92 × 32 × 8.2 mm |
No | £100–200 |
| OMRON complete ECG | OMRON | Yes | Yes | 90 readings | 232 × 123 × 98 mm | Yes – measures BP | £100–150 |
| Key: BP = blood pressure; ECG = electrocardiogram; iOS = iPhone operating system; OLED = organic light-emitting diode | |||||||
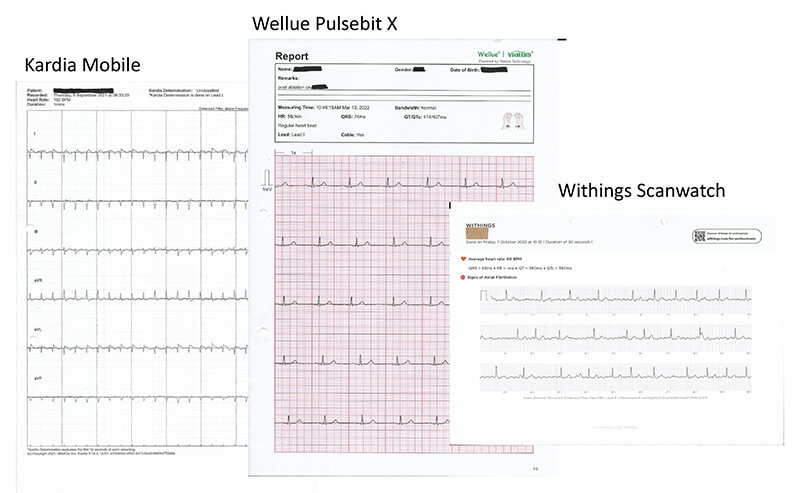
Discussion
 |
 |
 |
Both ECG and PPG technologies offer high diagnostic accuracies for AF, but only the devices recording an ECG can be used reliably for making a diagnosis of other arrhythmias. PPG technologies are of less use when trying to determine the cause of a paroxysmal tachycardia or bradycardia. Some helpful information can, however, be gained from PPG technologies in detecting that a pathological arrhythmia is present; very fast or slow rates of sudden onset and cessation, unassociated with changes in the patients’ activity, are pointers that a clinically significant arrhythmia is occurring.
AF, because of the irregularly, irregular pattern of the heart rate, is particularly suited to detection by PPG devices. Artificial intelligence (AI) in cardiology detects with a high accuracy using ECGs or PPG.3 In a recent meta-analysis1 examining the detection of AF, automatic detection by PPG had a sensitivity and specificity of 94.7% and 97.6%, respectively. While this makes PPG devices good for the detection of AF, the lack of an ECG trace makes identification of other more regular arrhythmias difficult.
Overall, devices providing an ECG recording have wider clinical applicability than PPG devices in determining the cause of different arrhythmias. They are also an excellent way of detecting AF and distinguishing them from similar atrial arrhythmias, such as typical and atypical atrial flutters, and, therefore, appear to be superior overall.
Cost and availability
While some patients will be in a position to purchase their own personal ECG recording devices, the cost will be an issue for many others. Loan of such devices by primary and secondary healthcare facilities for a sufficient time to allow capture of a repeatedly recurring symptomatic paroxysmal arrhythmia may be a valuable investment and cost-effective compared with the insertion of implantable loop recorders or repeated prolonged ambulatory ECG monitor recordings.
The provision of such devices in pharmacies, primary care, supermarkets and other venues frequently visited by high-risk populations has also been shown to be effective in detecting AF when it is either persistent or occurring in prolonged paroxysmal episodes.7 Such locally available options may be helpful for capturing symptomatic arrhythmias as well, if they are not especially transient.
It is important to recognise that the increasing availability of personal ECG-recording devices is not without problems. Abnormal results may cause anxiety, ECG misinterpretation may lead to overdiagnosis and overtreatment, ECG may detect other abnormalities (true or false positives) that may lead to invasive tests and treatments that have the potential for serious harm (e.g. angiography/revascularisation with bleeding, contrast-induced nephropathy and allergic reactions to the contrast). A structured plan for onward management of abnormalities detected on such devices needs to be formulated, and fundamental questions, such as what AF duration results in increased stroke risk and what risks are associated with non-sustained broad complex tachycardia detected by wearable monitoring devices, remain to be answered.
Conclusion
The increasing availability of personal and opportunistic ECG-recording devices and, to a lesser extent, PPG devices represents a very significant step forward in the care of patients presenting with symptomatic arrhythmias. New techniques for digital ECG analysis (e.g. machine learning and AI) and new technologies (e.g. wearables and injectables) have also opened up potentially significant opportunities for the detection and diagnosis of AF. These innovations may help to personalise therapy and risk stratification, but raise questions around whether previous clinical trials based on arrhythmias detected via non-wearable technologies are cross-applicable to arrhythmias detected by these devices. There is an urgent need to harness the potential of such technologies, and to help guide patients in making appropriate choices.
Key messages
- Portable electrocardiogram (ECG) recording devices, which patients can either manually activate themselves or program to automatically detect abnormal arrhythmias, allow important treatment to be started earlier
- Both non-12-lead ECG and photoplethysmography (PPG) technologies offer high diagnostic accuracies for atrial fibrillation (AF)
- An ECG recorded on a smart device is of sufficient quality for a cardiologist to review it and determine the diagnosis
Conflicts of interest
None declared.
Funding
None.
Note
The information about products was as publicly available at the time of article preparation.
References
1. Yang TY, Huang L, Malwade S, Hsu CY, Chen YC. Diagnostic accuracy of ambulatory devices in detecting atrial fibrillation: systematic review and meta-analysis. JMIR Mhealth Uhealth 2021;9:e26167. https://doi.org/10.2196/26167
2. Svennberg E, Sarkar S, Wouters F et al. Will smartphone applications replace the insertable cardiac monitor in the detection of atrial fibrillation? The first comparison in a case report of a cryptogenic stroke patient. Front Cardiovasc Med 2022;9:839853. https://doi.org/10.3389/fcvm.2022.839853
3. Wang YC, Xu X, Hajra A et al. Current advancement in diagnosing atrial fibrillation by utilizing wearable devices and artificial intelligence: a review study. Diagnostics (Basel) 2022;12:689. https://doi.org/10.3390/diagnostics12030689
4. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck app. JMIR Mhealth Uhealth 2019;7:e12284. https://doi.org/10.2196/12284
5. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa798
6. UK National Screening Committee. Screening in the UK: making effective recommendations 1 April 2019 to 31 March 2020. London: UK NSC, 2021. Available from: https://www.gov.uk/government/publications/uk-national-screening-committee-annual-report-2019-to-2020
7. Kamel Boulos MN, Haywood G. Opportunistic atrial fibrillation screening and detection in “self-service health check-up stations”: a brief overview of current technology potential and possibilities. Mhealth 2021;7:12. https://doi.org/10.21037/mhealth-19-204
8. Freedman B, Camm J, Calkins H et al. Screening for atrial fibrillation. Circulation 2017;135:1851–67.
9. Jonas DE, Kahwati LC, Yun JDY, Cook Middleton J, Coker-Schwimmer M, Asher GN. Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US preventive services task force. JAMA 2018;320:485–98. https://doi.org/10.1001/jama.2018.4190
10. Schnabel RB, Wallenhorst C, Engler D et al. Refined atrial fibrillation screening and cost-effectiveness in the German population. Heart 2022;108:451–7. https://doi.org/10.1136/heartjnl-2020-318882
