Despite widespread use of statins and other lipid-lowering therapies for hypercholesterolaemia, cardiovascular (CV) mortality and morbidity remains high. The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab and evolocumab, have been approved for use in patients with familial hypercholesterolaemia and high CV risk in the UK. We reviewed the records of patients at a large health board in Scotland, who were prescribed these agents, to determine their real-world efficacy and tolerability in routine clinical care.
Introduction
Hypercholesterolaemia, characterised by elevated serum total cholesterol and low-density lipoprotein (LDL), is a crucial factor for atherosclerosis and for the development of cardiovascular diseases (CVD). The hepatic protease proprotein convertase subtilisin/kexin type 9 (PCSK9) targets LDL-receptors for destruction.1,2 Removal of LDL from the blood stream is aided by increased expression of LDL-receptors.3
Statins have been proven to effectively lower LDL-cholesterol (LDL-C) levels and reduce CVD events in many high cardiovascular risk cohorts via 3-hydroxy-3-methylglutaryl coenzyme A (HMG Co-A) reductase inhibition. However, a significant number of patients with hypercholesterolaemia do not achieve their target LDL-C levels despite being on maximally tolerated statin therapy, while others are completely intolerant to statins.4,5
PCSK9 inhibitors (PCSK9i) have emerged as a promising therapeutic agent for treating hypercholesterolaemia and, more importantly, reduction of cardiovascular events. Currently available PCSK9is are either monoclonal antibodies that target and inactivate PCSK9 (alirocumab and evolocumab)6 or small-interfering RNA (siRNA) that inhibits the translation of PCSK9 from mRNA (inclisiran). The common pathway of action is the prevention of PCSK9-mediated LDL-receptor destruction. Statin therapy complements PCSK9i treatment in this regard by increasing LDL-receptor expression.7
Following the results of the landmark FOURIER (Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk) and ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trials, where PCSK9i have been shown to be efficacious in reducing serum total cholesterol and LDL-C, these agents have been approved for the treatment of high-risk patients in the National Health Service (NHS).8,9 Outside the confines of a clinical trial, data for the efficacy and tolerability of these agents in real-world routine clinical care is still evolving.
Method
We audited the indications, efficacy, and tolerability of PCSK9i prescribed for 62 patients by the NHS Tayside Cardiovascular Risk (CVR) Clinic between 2017 and 2021. The CVR clinic is a tertiary specialist clinic with a catchment area of approximately 415,000 NHS patients that is responsible for all PCSK9i prescribing in the health board area.
Efficacy was measured by analysing reductions in total cholesterol and calculated LDL-C at six and 18 months following prescription, from baseline (defined as the last cholesterol measurement prior to PCSK9i administration). We did this as LDL-C is a potent surrogate of cardiovascular (CV) events, hence our choice of looking at LDL-C lowering, which is also consistent with the mechanism of action of these drugs.
Statin tolerance status within the cohort was also observed, to determine if statin-intolerant patients reported lower LDL-C levels post-administration of PCSK9i.
Comparative analysis was also made for patients administered with alirocumab and evolocumab to check for a difference in efficacy between the two agents. Similarly, data were analysed to compare LDL-C reduction in patients with familial hypercholesterolaemia (FH) and patients with polyvascular disease, the two currently approved indications for these agents in the UK. At the time of the audit, inclisiran had not been approved for clinical use.
Results
Table 1. Indications for administration of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i)
| Indication | Number (%) |
|---|---|
| Heterozygous FH | 22 (35) |
| Polyvascular disease | 31 (50) |
| Deemed as having phenotypic FH | 8 (13) |
| High lipoprotein (a) | 1 (2) |
| Total patients administered PCSK9i | 62 (100) |
| Key: FH = familial hypercholesterolaemia | |
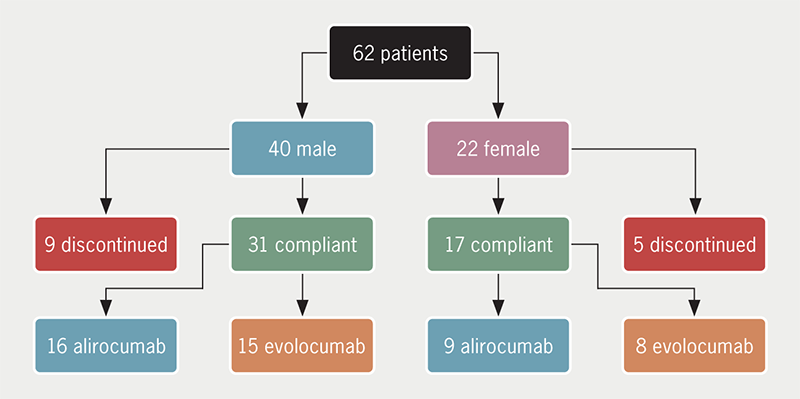
There were 62 patients, 40 males and 22 females, who were prescribed a PCSK9i (31 alirocumab and 31 evolocumab) over a five-year period, although clinical contact was significantly reduced between March 2020 and December 2020, due to the pandemic. The indication for PCSK9i was heterozygous FH in 22/62 patients and polyvascular disease in 31/62. Eight of the remaining nine patients were treated on the basis of having been deemed as having phenotypic FH (genetics negative) by their treating clinician, while the last was a patient with high lipoprotein (a) who was commenced on PCSK9i therapy by a cardiology multi-disciplinary team, following admission for myocardial infarction (table 1).
A total of 14/62 (9/40 males and 5/22 females) discontinued therapy (figure 1). Four of these discontinued due to non-compliance. In two cases, compliance was not ascertainable due to insufficient data and seven had various reported side effects, including arthralgia, myalgia, rash, rhinosinusitis, sore throat, abdominal pain, diarrhoea, back pain, headaches and palpitations. The majority of the aforementioned side effects were not specified in the clinical trials for these agents, making comparisons inappropriate, although the one patient (1.6%) who discontinued due to rash and the four who discontinued due to myalgia (6.4%), are not dissimilar from the percentage of patients who developed “allergic reaction” (3.1–7.9%) and “muscle-related event” (5%) in the FOURIER and ODYSSEY trials.8,9 In one patient, total cholesterol and LDL-C increased on PCSK9i compared with statin therapy and this was thought to be due to their specific LDL-receptor mutation (heterozygous for LDL-receptor c.313+1G>C). Eleven out of the 14 patients who discontinued PCSK9i therapy were also intolerant of statins, which included all seven who reported side effects on PCSK9i. There were 33/62 classed as statin intolerant, of whom 26/33 tolerated PCSK9i agents, while seven discontinued therapy as stated previously.
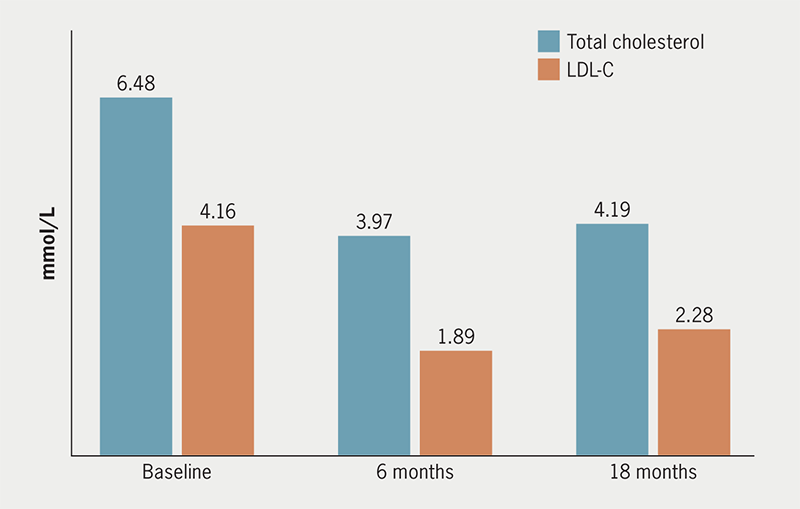
Absolute values of total cholesterol and LDL-C at baseline and after six and 18 months for the 48 patients taking PCSK9i showed significant reductions in cholesterol levels (figure 2).
Percentage reductions from baseline cholesterol concentrations at six and 18 months, in this group and specific subgroups of patients, can be seen in table 2. Patient numbers were comparable among subgroups with the exception of female and male distribution (with there being almost twice as many males than females). The lipid-lowering effects seen were similar to each subgroup, with LDL-C concentrations at six and 18 months reduced by 45–55%. After 18 months, both monitoring and adherence deteriorate slightly.
Table 2. Total and low-density lipoprotein-cholesterol (LDL-C) percentage reductions from baseline at 6 and 18 months after PCSK9i therapy in patient subgroup (n denotes number of patients in each subgroup)
| Baseline, mmol/L | 6-month reduction | 18-month reduction | |
|---|---|---|---|
| All (n=48) | |||
| Total cholesterol | 6.48 | 39% | 35% |
| LDL-C | 4.16 | 55% | 45% |
| Statin intolerant (n=26) | |||
| Total cholesterol | 6.91 | 37% | 37% |
| LDL-C | 4.69 | 55% | 46% |
| Alirocumab (n=25) | |||
| Total cholesterol | 6.31 | 34% | 33% |
| LDL-C | 3.84 | 46% | 41% |
| Evolocumab (n=23) | |||
| Total cholesterol | 6.55 | 43% | 37% |
| LDL-C | 4.4 | 63% | 48% |
| Female (n=17) | |||
| Total cholesterol | 6.54 | 30% | 31% |
| LDL-C | 4.39 | 50% | 38% |
| Male (n=31) | |||
| Total cholesterol | 6.36 | 43% | 38% |
| LDL-C | 3.77 | 54% | 48% |
| Familial hypercholesterolaemia (n=18) | |||
| Total cholesterol | 5.83 | 34% | 32% |
| LDL-C | 3.95 | 54% | 44% |
| Polyvascular disease (n=23) | |||
| Total cholesterol | 6.9 | 39% | 36% |
| LDL-C | 4.6 | 55% | 45% |
A large proportion of patients (>50%) taking PCSK9i who are intolerant of statins and have high CVD risk experience substantial and sustained lipid-lowering effect from these agents. The percentage reductions seen would not be achievable on other conventional therapies.
In comparison, between the two PCSK9is, evolocumab appears to exert a greater lipid-lowering effect than alirocumab. This observation could be due to the different doses of alirocumab available: within this cohort, 14 patients were prescribed 75 mg and 11 patients were prescribed 150 mg once every two weeks. We observed a trend of greater total cholesterol lowering among the subgroup who were prescribed the 150 mg dose after six months of therapy (36% reduction for 150 mg compared with 27% reduction for 75 mg). This was not statistically significant in our data but fits with the observation made by Kastelein et al. when they interrogated data from the ODYSSEY trials and found an additional 14.2% decrease in LDL-C following introduction of the larger dose of alirocumab.10
We observed that males derived greater benefit from PCSK9i therapy than females, and an unpaired t-test showed a significant difference (p=0.0078) between the total cholesterol concentration of men and women after six months of therapy, although this was not observed after 18 months or for LDL-C, due to fewer data points at 18 months. This contrasts with the findings of the major pivotal trials. This could likely be due to the greater variability from the smaller sample size rather than due to any differential pharmacological effect.
Indication for treatment and aetiology of disease had no bearing on the cholesterol-lowering effect of PCSK9i as both FH and PVD patients displayed similar cholesterol reductions after six and 18 months.
Conclusion
Our analysis confirmed the efficacy of PCSK9i in real-world clinical practice with the efficacy outcomes similar to the pivotal trials of these agents. ODYSSEY reported a 54% reduction in LDL-C compared with baseline after six months of alirocumab therapy; for FOURIER and evolocumab, this was 61%.8,9 Our data showed reductions of 46% and 63% in LDL-C compared with baseline, for alirocumab and evolocumab, respectively. A key observation of our study was the strong, sustained, and comparable cholesterol reductions observed between high-risk statin-intolerant patients and other patients with known CVD risks. While males and patients on evolocumab derived greater benefit, dose equivalences and increased sample sizes would be required to confirm this.
A limitation of our audit was the relatively small sample size. However, it does accurately reflect the total uptake of these agents in real-world practice and the impact the pandemic has had as the CVR clinic is the sole prescriber of these agents for the region. Also, non-adherence to PCSK9i for long-term treatment may have contributed to incomplete data in a few excluded patient records.
Despite favourable indications confirming efficacy in real-world practice, future analysis of clinical data will help validate the observations made here and clarify the implications of prolonged use of PCSK9i on reducing cardiovascular morbidity, particularly in less studied populations, such as statin-intolerant patients.
Key messages
- Familial hypercholesterolaemia and high cardiovascular risk patients administered with proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) showed significant reductions in absolute values of total cholesterol and low-density lipoprotein-cholesterol (LDL-C)
- Efficacy of the PCSK9i agents is evident in clinically challenging cohorts, such as statin-intolerant patients
- Future analysis of clinical data from larger datasets will help clarify the implications of prolonged use of PCSK9i on reducing cardiovascular morbidity, particularly in lesser-studied cohorts, such as statin-intolerant patients
Conflicts of interest
None.
Funding
None.
Study approval
Necessary Caldicott approval for this audit was obtained from NHS Tayside.
References
1. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72. https://doi.org/10.1056/NEJMoa054013
2. Abifadel M, Varret M, Rabès J-P et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–6. https://doi.org/10.1038/ng1161
3. Ji E, Lee S. Antibody-based therapeutics for atherosclerosis and cardiovascular diseases. Int J Mol Sci 2021;22:5770. https://doi.org/10.3390/ijms22115770
4. Brugts JJ, Yetgin T, Hoeks SE et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376. https://doi.org/10.1136/bmj.b2376
5. Iyen B, Akyea RK, Weng S, Kai J, Qureshi N. Statin treatment and LDL-cholesterol treatment goal attainment among individuals with familial hypercholesterolaemia in primary care. Open Heart 2021;8:e001817. https://doi.org/10.1136/openhrt-2021-001817
6. Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: a new era of lipid lowering therapy. World J Cardiol 2017;9:76–91. https://doi.org/10.4330/wjc.v9.i2.76
7. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009;29:431–8. https://doi.org/10.1161/ATVBAHA.108.179564
8. Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. https://doi.org/10.1056/NEJMoa1615664
9. Schwartz GG, Steg PG, Szarek M et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. https://doi.org/10.1056/NEJMoa1801174
10. Kastelein JJ, Kereiakes DJ, Cannon CP et al. Effect of alirocumab dose increase on LDL lowering and lipid goal attainment in patients with dyslipidemia. Coron Artery Dis 2017;28:190–7. https://doi.org/10.1097/MCA.0000000000000438
