Dear Sirs,
We read with interest the cross-sectional study by Kazemi et al. exploring the cardiovascular phenotypes of 690 hospitalised COVID-19 patients in a tertiary centre in Iran. The authors found the prevalence of new-onset hypertension (as defined by a systolic blood pressure [BP] ≥130 mmHg and/or diastolic BP ≥80 mmHg) was 10%.1 Longer-term follow up of these patients and documentation of electrolyte dysfunction are lacking and, with due respect, are limitations of their work.
In the midst of the first wave of the COVID-19 pandemic in the United Kingdom, we observed new-onset systolic hypertension associated with hypernatraemia and hypokalaemia with normal serum urea among hospitalised patients. We investigated two patients and found elevated urinary potassium (without causal drugs) and hyporeninaemic hypoaldosteronism (plasma renin <0.2 nmol/L/hr [reference range: 0.5–3.5 nmol/L/hr] and aldosterone <60 pmol/L [reference range: 60–250 pmol/L]) in both. Congenital and secondary endocrine causes of hypertension were excluded.
We treated one of the patients with amiloride, which resulted in normalisation of serum/urinary potassium and BP within one week (figure 1). Plasma renin and aldosterone levels normalised after three weeks when amiloride was withdrawn.2
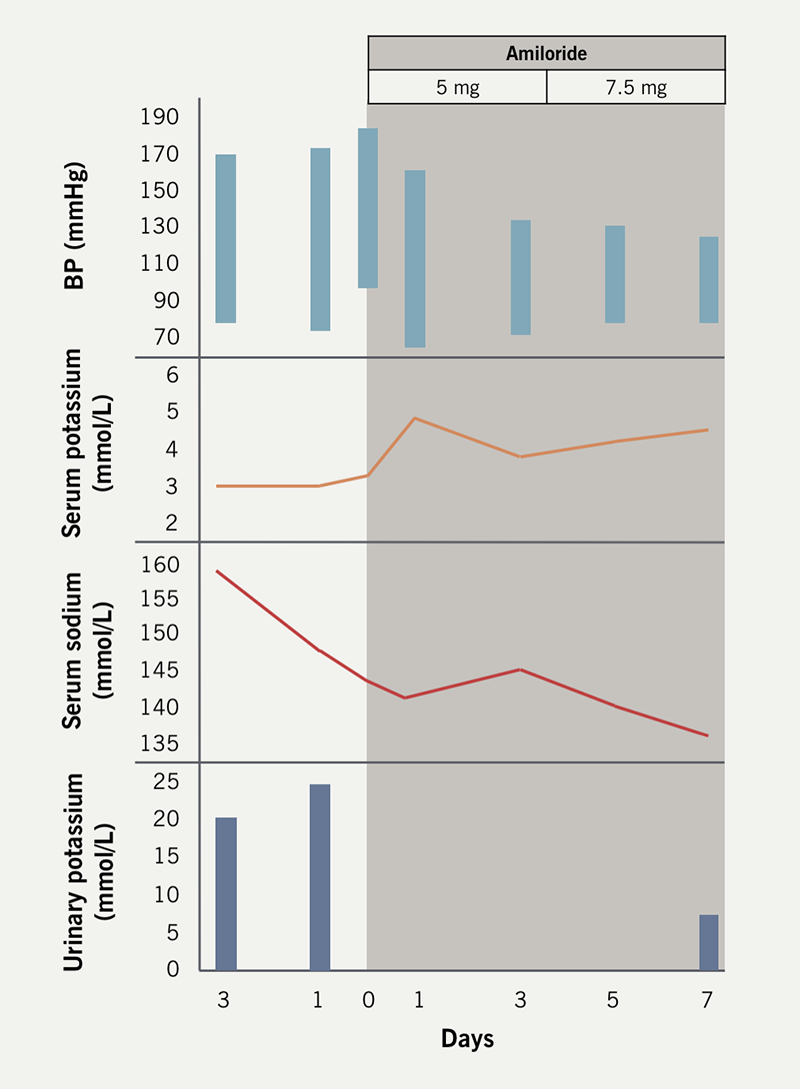
We suspect that transient hyporeninaemic hypoaldosteronism may be related to dysregulated sodium channel (ENaC) pathophysiology, similar to that in Liddle’s syndrome. Correction of electrolyte abnormalities and reversal of hypertension with the diuretic amiloride, which inhibits sodium reabsorption by selectively blocking this channel,3,4 supports this reasoning. The disease entity appears to be self-limiting and contained within the period of acute infection.
Normalisation of the renin-angiotensin-aldosterone system (RAAS) during convalescence may reflect a direct effect of COVID-19 on ENaC homeostasis leading to a distal tubulopathy with renal potassium wasting related to angiotensin-converting enzyme (ACE) 2 degradation. Chen et al. hypothesised that reduced counteractivity of ACE2 against ACE1 resulted in disordered RAAS activity and that the end of renal potassium loss reflected the end of the disruption on the RAAS,5 which our findings support.
This ‘Liddle’s-like’ picture in COVID-19 may be adequately explained by the downregulation of ACE2 from viral binding resulting in upregulation of angiotensin II, which enhances ENaC activity in the distal nephron.6 This leads to hypertension and hypokalaemia which suppress the RAAS, resulting in hyporeninaemic hypoaldosteronism. As described, amiloride or the pharmacologically similar agent triamterene could be trialled.
Amit KJ Mandal
Consultant Physician
(amit.mandal@nhs.net)
Jason Kho
Specialist Registrar
Departments of Medicine and Cardiology, Wexham Park Hospital, Frimley Health NHS Foundation Trust, Wexham Street, Slough, SL2 4HL
Constantinos G Missouris
Consultant Physician and Professor of Cardiology
Wexham Park Hospital, Frimley Health NHS Foundation Trust, UK; University of Nicosia Medical School, Nicosia, Cyprus
Conflicts of interest
None declared.
Funding
None.
References
1. Kazemi E, Daliri S, Chaman R et al. Cardiovascular disease development in COVID-19 patients admitted to a tertiary medical centre in Iran. Br J Cardiol 2024;31:79. https://doi.org/10.5837/bjc.2024.026
2. Mandal AKJ, Kho J, Metaxa S et al. COVID-19 and late-onset hypertension with hyorininaemic hypoladosteronism. Int J Clin Pract 2020;75:e13773 https://doi.org/10.22541/au.160133530.08134398
3. Synder PM, McDonald FJ, Stokes JB et al. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 1994;269:24379–83
4. Enslow BT, Stockand JD, Berman JM. Liddle’s syndrome mechanisms, diagnosis and management. Integr Blood Press Control 2019;12:13–22. https://doi.org/10.2147/IBPC.S188869
5. Chen D, Li X, Song Q et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open 2020;3:e2011122. https://doi.org/10.1001/jamanetworkopen.2020.11122
6. Zaika O, Mamenko M, Staruschenko A et al. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr Hypertens Rep 2013;15:17–24. https://doi.org/10.1007/s11906-012-0316-1
