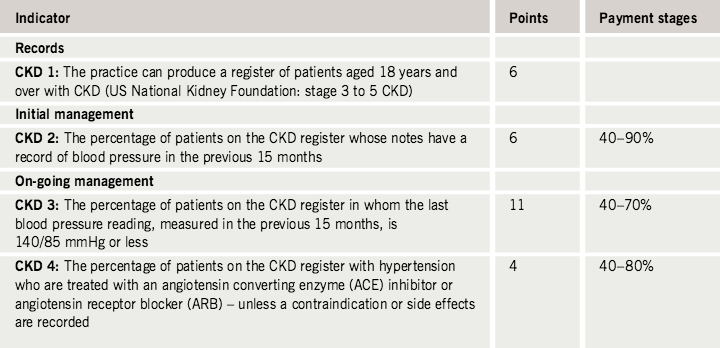
Chronic kidney disease (CKD) is an independent risk factor for cardiovascular disease and in February 2006 was added to the Quality and Outcomes Framework (QOF) for primary care in the UK. The QOF indicators apply to all patients with stage 3–5 CKD and include the production of a register of such patients, appropriate monitoring and treatment of hypertension and the prescription of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs).
Introduction
Chronic kidney disease (CKD) is an independent risk factor for cardiovascular disease.1 Almost half of all deaths in patients with CKD are caused by cardiovascular events2 and, in diabetic subjects, mortality increases significantly with reduced kidney function.3 As part of the National Service Framework for Renal Services, CKD was added in February 2006 to the Quality and Outcomes Framework (QOF) for primary care in the UK. Within this, up to 27 points can be earned for the production of a register of patients with stage 3–5 CKD and the appropriate monitoring and treatment of hypertension and prescription of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in these patients (table 1).
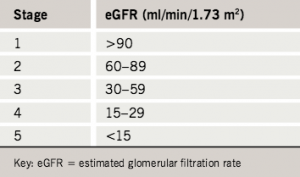
A key feature of these guidelines is that instead of using serum creatinine concentration as a surrogate measure of glomerular filtration rate (GFR), as has been widely used in the past, they use the Modification of Diet in Renal Disease (MDRD) equation, which uses serum creatinine and the age, sex and ethnicity of each patient to calculate an estimated glomerular filtration rate (eGFR). The eGFR can then be used to group patients into one of the five stages of CKD developed by the US National Kidney Foundation (table 2).
The QOF indicators apply to those patients in stages 3–5 CKD. The range of serum creatinine concentrations corresponding to stage 3 CKD using this MDRD equation in Caucasian females (a) and males (b) between the ages of 20 and 100 years is shown in figure 1. This demonstrates that using only serum creatinine concentration leaves a substantial number of patients with stage 3 disease undiagnosed. This is supported clinically by a recent study in Salford, which showed that 80% of women with diabetes and stage 3 CKD have serum creatinine < 120 µmol/L.4

In the past, the use of creatinine alone has therefore missed the opportunity to detect important reductions in GFR with the result that patients with early disease have not received appropriate management of their blood pressure and prescription of an ACE inhibitor or ARB. These drugs have the potential to slow the progression of renal disease, as well as influence cardiovascular risk factors, providing the potential to prevent cardiovascular events. The hope is that the introduction of these guidelines within primary care will promote earlier diagnosis and treatment of complications as well as slow or prevent the progression to more serious CKD and reduce the risk of major illness or death due to cardiovascular disease. The practical implementation, however, raises a number of issues in primary care. It is expected that following these guidelines up to an additional 1.4% of the population will be diagnosed with CKD5 and, as laboratories begin to routinely include an eGFR alongside creatinine measurements, it is likely that many of these new diagnoses of CKD will result from routine investigations. Treating all patients with an eGFR < 60 ml/min/1.73m2 will also mean starting an ACE inhibitor or ARB in large numbers of elderly patients whose decline in renal function has, in many cases, previously been attributed to normal physiological age-related decline. We, therefore, looked at one representative primary care practice in Hertfordshire and asked:
- How many patients can a typical primary care practice expect to have on the
CKD register? - How many of these patients are already identified as having CKD?
- How many of these patients are already being treated to target?
- When should an ACE inhibitor or ARB not be prescribed?
- How should patients newly diagnosed with stage 3–5 CKD be managed in primary care?
- How much will this additional care cost?
How many patients can a typical primary care practice expect to have on the CKD register?
The practice has five general practitioners (GPs) and serves 9,021 patients from a predominantly Caucasian population of mixed socio-economic demography. Using age-based criteria (table 3), 963 patients (10.7% of the practice population) with stage 3–5 CKD were identified. It is worth noting that this is double the estimated prevalence across the UK.6 The breakdown of age, sex and stage of these patients is shown in table 4 and the percentage practice population at each age group with stage 3–5 disease plotted in figure 2. In agreement with previous studies, the prevalence increases with increasing age and is greater in females than males at all ages.5,7
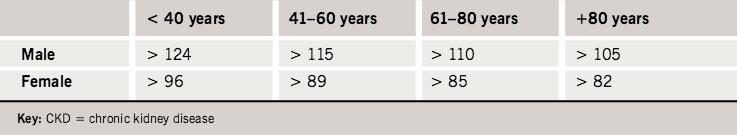
ages to identify all patients with stage 3–5 CKD

How many of these patients are already identified as having CKD?

Despite the recent increased interest in CKD, only 148 of these 963 patients (15.4%) had been added to the CKD register during routine clinical care in the first eight months following the introduction of the QOF guidelines in February 2006. Two of those had no previous serum creatinine concentrations recorded but of the remaining 146 patients, 112 (76.7%) were stage 3, 31 (21.2%) were stage 4 and 3 (2.1%) were stage 5. This supports recent reports that only a small proportion of the UK population with stage 3–5 CKD are recorded as having renal disease.5
At least part of this under-reporting is probably due to previous use of a serum creatinine concentration > 125 µmol/L as the threshold for further investigation of renal function. Using this threshold identified 267 patients, of whom 265 (175 male and 90 female) were stage 3–5 (216 [81.5%] stage 3, 43 [16.2%] stage 4 and 6 [2.3%] stage 5). Taking into account changes in renal function during analysis, the percentage of the total practice prevalence of stages 3, 4 and 5 already on the CKD register and identified by a serum creatinine > 125 µmol/L are shown in figure 3a. As expected from figure 1, using serum creatinine > 125 µmol/L as the threshold identifies all patients with stages 4 and 5 CKD but only a small proportion (24%) of those with stage 3 disease. Figure 3b shows the percentage of patients with stage 3 disease with a serum creatinine concentration > 125 µmol/L and demonstrates that, again as expected, the majority of this under-diagnosis occurs in female patients.

How many of these patients are already being treated to target?
When the QOF targets were introduced it was suggested that approximately 90% of patients with stage 3–5 CKD would already be on another cardiovascular disease register and so would already be on medication for blood pressure. Given the large numbers of patients involved, detailed analysis was limited to the 265 patients with a serum creatinine of > 125 µmol/L. Of these, 182 (68.7%) were also on the hypertension register, 30 (11.3%) on the stroke register, 72 (27.2%) on the diabetes register and 84 (31.6%) on the coronary heart disease register, leaving only 37 (14.0%) not on any other cardiovascular risk disease register. Two hundred and forty-six (92.8%) had a blood pressure recorded within the last 15 months and, of those, 181 (68.3%) had a blood pressure at or below the target of 140/85 mmHg. Of the others, three patients had been excepted from the hypertension indicators, leaving 69.1% of suitable patients with a creatinine > 125 µmol/L with a blood pressure reading in the previous 15 months of 140/85 mmHg or less. Notably, of the other 62 patients with a blood pressure > 140/85 mmHg, 56 were already on the hypertension register. However, only 147 (55%) of these 265 patients with a creatinine > 125 µmol/L were taking an ACE inhibitor or ARB.
When should an ACE inhibitor or ARB not be prescribed?

an angiotensin-converting enzyme
(ACE) inhibitor or angiotension
receptor blocker (ARB)
The recommendation to prescribe an ACE inhibitor or ARB to patients with stage 3–5 CKD follows reports that such treatment can slow the progression of renal disease. The strongest evidence comes from studies in patients with diabetic nephropathy where one study found that over four years ACE inhibitor treatment would save 195 life-years per 1,000 patients8 and, using the same data, another study found that ACE inhibitor treatment would save 21.18 dialysis-years per 100 patients over the four years studied.9 However, the results of a meta-analysis suggested that ACE inhibitors also have a substantial beneficial effect in delaying the onset of end-stage renal failure in non-diabetic patients with renal disease10 and such patients taking ACE inhibitors were found to have about a 30% lower risk of developing end-stage renal failure than patients receiving other antihypertensive agents.11 Despite this, there are patients in whom it is not appropriate to prescribe an ACE inhibitor or ARB, either because of specific contraindications, additional medical conditions or circumstances surrounding their care. Of particular note, many older patients already being treated for hypertension are on calcium channel blockers and diuretics, as per the National Institute for Health and Clinical Excellence (NICE) guidance for patients over 55 years old, and adding an ACE inhibitor needs to be carefully considered in view of the risk of hypotension, particularly when accompanied by dehydration in the summer and during cases of diarrhoea and vomiting. Accordingly, in patients over 55 years in whom blood pressure is well controlled with a diuretic and calcium channel blocker it is not appropriate to add an ACE inhibitor unless proteinuria is present. It is therefore reasonable to exception report such patients. A list of these specific contraindications is given in box 1.
How should patients newly diagnosed with stage 3–5 CKD
be managed in primary care?
Although treatment of blood pressure and the prescription of an ACE inhibitor or ARB are the only indicators included in the QOF guidelines they are not the only considerations when managing patients with CKD. Particularly important is the appropriate assessment of the increasing number of patients with an eGFR < 60 ml/min/1.73m2 not previously known to have kidney disease. Box 2 outlines a suggested initial assessment for such patients (for more detail see references 14 and 15).


Particular attention is drawn to the importance of dipstick testing of urine because, when kidney function is impaired, proteinuria is associated with an increased risk of cardiovascular events that persists after adjustment for eGFR and is independent of diabetic status.16 The Cholesterol and Recurrent Events (CARE) trial investigators examined the effect of proteinuria, impaired kidney function and adverse outcomes in patients with coronary disease and showed that a higher risk of mortality was associated with heavier proteinuria on dipstick urinalysis and lower kidney function, and the risk associated with these conditions was additive. In patients without diabetes, microalbuminuria is an important risk factor for cardiovascular end points and patients in whom proteinuria was decreased using ramipril showed a reduced incidence of cardiovascular events.17 This was highlighted in the recent UK Consensus Conference on Early Chronic Kidney Disease which recommended sub-classifying CKD stage 3 into two groups – 3A and 3B – depending on the eGFR and adding a suffix p to all stages to reflect the risk of progressive kidney disease in patients with proteinuria.18 If the early morning proteinuria:creatinine ratio is > 100 mg/mmol, blood pressure should also be treated at >130/80 mmHg to a lower target of <125/75 mmHg with inclusion of an ACE inhibitor or ARB.12
How much will this additional
care cost?
For the average primary care practice with average prevalence the 27 points available from the renal QOF will produce a payment of £3,364. Savings will also be made in the longer term by preventing deaths from cardiovascular disease and other complications from renal disease but, in the short term, the introduction of these new guidelines place a significant workload on GPs. Accordingly, most of the cost of implementing these guidelines is in terms of time.
This includes the time to:
- calculate eGFR until it is routinely
reported by laboratories - review the practice database
- review patients who need review and recall
- arrange follow-up visits and blood tests if an ACE inhibitor is started
- convert to ARB and re-check urea and electrolytes if ACE inhibitor intolerant.
Conclusion
Although considerable time has already been invested in this, and there is still much work to be done to complete the reviews of the target practice population of 963 patients with stage 3–5 CKD, the implementation of the QOF guidelines have resulted in an accurate practice-based register for CKD and identified a number of issues surrounding the management of these patients. The creation of the register was facilitated by the 2006 kidney dialysis outcomes quality initiative, which classified the stages of CKD according to eGFR19 and provides the essential first step for any chronic disease management. The task now is to maintain an up-to-date register and to recall and regularly review the patients. While it is important to remember the very small minority of patients who will progress to CKD stage 5 and who need to be detected early and appropriately referred, for the majority of patients the major benefit of this improved patient management will be cardiovascular risk reduction. As most patients with CKD already have cardiovascular co-morbidities it may be sensible to manage the care of these patients in much the same way as is already being performed for coronary heart disease, hypertension, diabetes and stroke. This might also provide an opportunity to combine these registers and generate one cardiovascular risk clinic with separate protocols and blood pressure targets for patients on more than one register. An added advantage of this would be the avoidance of the anxiety provoking CKD label. Establishing such a system will inevitably take time but, in the meantime, identifying patients with stage 3–5 CKD and following a simple initial management protocol such as that described in box 2 can only lead to improved patient care.
Conflict of interest
MK has received financial support for research from pharmaceutical companies in the past including honoraria for speaking at conferences and travel expenses from Pfizer, MSD, Astellas, Takeda, GSK, Bayer and Lilly. JU-S, AY and SC: none declared.
Key messages
- Primary care practices can expect up to 5% of their practice population to have stage 3–5 chronic kidney disease (CKD)
- Practices that have a high proportion of elderly patients may have a prevalence of up to 10%
- The majority of patients with CKD can be managed within primary care and for them the major benefit of care will be cardiovascular risk reduction
- Initial assessment of all patients with an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 not previously known to have kidney disease should include at least a full history, a focused clinical examination, full blood count, urea and electrolytes, fasting lipids, calcium, phosphate, parathyroid hormone (if calcium is abnormal) and urine dipstick
- There are a number of patients in whom it is not appropriate to prescribe an ACE inhibitor or angiotensin receptor blocker despite CKD and particular consideration needs to be given to the elderly who do not have proteinuria and in whom the risk/benefit ratio is uncertain
References
- Coresh J, Astor B, Sarnak M. Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 2004;13:73–81.
- Wali R, Henrick W. Chronic kidney disease: a risk factor for cardiovascular disease. Cardiol Clin 2005;23:
343–62. - Nag S, Bilous R, Kelly W et al. All-cause and cardiovascular
mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years’ data from the South Tees Diabetes Mortality study. Diabet Med 2007;24:10–17. - Middleton R, Foley R,
Hegarty J et al. The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant 2006;21:88–92. - de Lusignan S, Chan T, Stevens P et al. Identifying patients with chronic kidney disease from general practice computer records. Fam Pract 2005;22:234–41.
- John R, Webb M, Young A et al. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis 2004;43:825–35.
- Coresh J, Astor B, Greene T et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12.
- Hendry B, Viberti G, Hummel S et al. Modelling and costing the consequences of using an ACE inhibitor to slow the progression of renal failure in type I diabetic patients. QJM 1997;90:277–82.
- Garattini L, Brunetti M, Salvioni F et al. Economic evaluation of ACE inhibitor treatment of nephropathy in patients with insulin-dependent diabetes mellitus in Italy. Pharmacoeconomics 1997;12:67–75.
- Giatras I, Lau J, Levey A. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med 1997;127:337–45.
- Ruggenenti P, Perna A, Remuzzi G. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol 2001;12:2832–7.
- British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, Stroke Association. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 2005;91(suppl 5):v1–52.
- Fogari R, Preti P, Derosa G et al. Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur J Clin Pharmacol 2002;58:177–80.
- Royal College of General Practitioners. Introducing eGFR, promoting good CKD management. London: RCGP.
- McKie Kane-ToddHall SMBJ. Chronic kidney disease and eGFR. Update 2006;August:27–32.
- Anavekarn S, Gans D, Berl T et al. Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension, case for albuminuria. Kidney Int 2004;92(suppl):S50–5.
- Schrader J, Luders S, Kulschewski A et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension. J Hypertens 2006;24:541–8.
- Archibald G, Bartlett W, Brown A et al. UK consensus on early chronic kidney disease. 2007.
- KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kid Dis 2002;39(2 suppl 2):S1–246.
