Stent thrombosis (ST) is an uncommon but serious complication of percutaneous coronary intervention (PCI), and is associated with the discontinuation of antiplatlet therapy. In a retrospective study of ST cases during a two-year period of the Wessex Regional Cardiac Unit, 3,004 (1,661 emergency and 1,343 elective) patients underwent PCI between November 2003 and October 2005. There were 25 episodes of ST occurring in 22 patients (overall incidence of ST is 0.83%). There were two (8%) cases of acute ST, eight (32%) of sub-acute ST and 15 (60%) of late or very late ST (five cases between six and 12 months and one case more than one year post-procedure). In the late and very late ST group only one patient was taking dual antiplatelet therapy.
Introduction
Stent thrombosis (ST) is a potentially life-threatening complication of coronary artery stent placement (percutaneous coronary intervention [PCI]). Concern about the incidence of ST is greatest in patients treated with drug-eluting stents (DES), in whom some evidence suggests there is a higher incidence than for bare metal stents (BMS).1–3 Recently reported meta-analyses from collections of randomised studies comparing BMS with either Cypher® or Taxus® DES have been interpreted as confirming this increase in risk for these drug-eluting devices.4 It is likely that ST is multi-factorial in its aetiology since evidence demonstrates that there are procedural factors associated particularly with earlier ST, but cessation of antiplatelet therapy has also been identified as a significant risk factor.5–7 The role of relative hypo-responsiveness to aspirin and clopidogrel remains unclear but some data suggest that this is an aetiological factor in at least some cases.8
The aim of this study was to identify the incidence of ST in a large cohort of consecutive cases and specifically to assess the timing of each ST case in relation to antiplatelet therapy status at the time of the event.
Methods
Data were derived from a retrospective analysis from a consecutive series of patients undergoing PCI at the Wessex Cardiac Unit between November 2003 and October 2005. Cases were identified by the following methods: (a) a search of the hospital database exploring in-patient discharge summaries using the search term ‘stent thrombosis’ followed by analysis of every case derived from this search; (b) clinical PCI database; (c) post-mortem records. Once identified, all cases of ST were analysed to produce a data-set that included, demographics, clinical urgency (elective, urgent, emergency), procedural details (number of treated vessels, number of stents implanted, BMS or DES, stent length, etc.), time of index event from procedure, antiplatelet therapy (aspirin alone, aspirin plus clopidogrel, clopidogrel alone, or neither), ST treatment, and clinical outcome. The new ‘Dublin definitions’ have been used to classify ST cases.
Results
Of 3,004 consecutive cases treated between November 2003 and October 2005, 1,661 (55%) were non-elective and 1,343 (45%) were elective. The DES utilisation rate during this period was 90.0% of all stents. There were 25 cases of ST occurring in 22 patients, mean age 58 (range 42–82) years (19 men and six women), thus giving an overall incidence of 0.83% (1.02% in non-elective and 0.6% in elective cases). Simultaneous ST in more than one vessel in the same patient is counted as a single episode. In the ST group, mean stent length was 30.2 (range 16–116) mm and mean stent diameter 2.77 (range 2.25–4.0) mm. Twenty-four of the cases were in DES (96%) and one case was in BMS. The diagnosis of ST was made angiographically in 20 cases (80%), clinically (history with electrocardiogram [ECG] and cardiac enzymes) in four cases (16%) and at post-mortem in the remaining case.
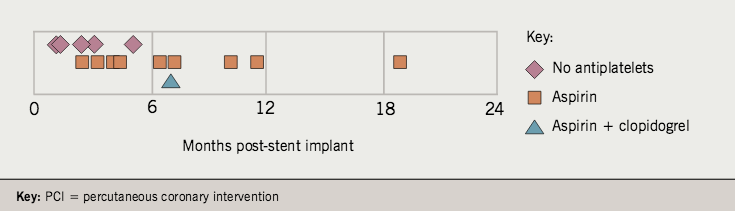
According to the Dublin definitions, the time distribution of ST cases was as follows: (a) acute – two cases (8%); (b) subacute – eight cases (32%); (c) late – 14 cases (56%); (d) very late – one case (4%). Of the 15 cases of ST presenting as late or very late, the mean time from procedure was 174 (range 39–537) days (figure 1).
Antiplatelet therapy
Until early 2005, the policy in this unit was aspirin for life with clopidogrel for six months post-PCI. In 14 of the 15 cases presenting as late or very late ST, clopidogrel had been stopped. In 10 cases (66% of this group), the patient was on aspirin alone; four patients (27%) were on no antiplatelet therapy at all (figure 1). Of patients who had stopped antiplatelet therapy, identifiable reasons were: advice of the attending cardiologist in seven cases and advice of the general practitioner in one case. Antiplatelet therapy had been discontinued against medical advice in
six cases.
Outcome
 Ninety-six per cent of patients with ST experienced non-fatal myocardial infarction and one patient (4%) died. In 19 cases (76%) the patients were treated by repeat PCI (plain balloon angioplasty in nine cases and a further stent in the remaining 10). TIMI III flow was established in 17 cases.
Ninety-six per cent of patients with ST experienced non-fatal myocardial infarction and one patient (4%) died. In 19 cases (76%) the patients were treated by repeat PCI (plain balloon angioplasty in nine cases and a further stent in the remaining 10). TIMI III flow was established in 17 cases.
Discussion
This study provides some valuable information about the pattern of ST and relation to antiplatelet therapy in a large ‘real world’ cohort of consecutive patients derived from a centre with a 90% DES rate. Firstly, 60% (15) of the cases occurred either ‘late’ or ‘very late’ according to the new Dublin definitions, with a mean time from procedure of 174 (range 39–537) days. Furthermore, in only one of these 15 cases was the patient still taking dual antiplatelet therapy, clopidogrel having been discontinued in 14 of the 15. The reasons for stopping clopidogrel were variable, since the policy at this centre for most of the study period was to recommend dual antiplatelet therapy for only six months, but it is reasonable to speculate that the absence of clopidogrel in this population may have contributed to the ST event, particularly in the light of previous data.5–7 The aetiology of this complication is likely to be multi-factorial, and it is of interest that the mean stent length of our ST population was 30.2 mm, a risk factor that is previously reported.9 This risk factor may be directly linked to that of delayed endothelialisation.10–12
This study highlights an important limitation inherent in the assessment of ST in any centre, that is, the ability to confidently capture all cases. Despite our triple-check methodology, and despite the overall ST rate we report being compatible with previous data, it is inevitable that we have not identified a proportion of ST cases that occurred in our population of 3,004 consecutive patients. Some patients with ST will have presented to other centres and been treated medically, or will have been diagnosed with another condition. It is also possible that some cases may have remained asymptomatic or have died without the diagnosis ever having been made. These possibilities put into perspective the apparently relatively good outcome in the population that we have reported in which only one patient died. These patients are likely to be a selected group. Meticulous study of the incidence and aetiology of ST therefore now demands a central registry that encourages reporting from all cardiology specialists in order to track more accurately the true ST event rate, and its outcome.
Despite this limitation, our data suggest that any attempts to minimise the incidence of ST should be dominated by scrupulous attention to the policy relating to antiplatelet therapy. This study adds some weight to the prevailing convention that dual antiplatelet therapy should be continued for at least one year. Furthermore, these data suggest that greater focus should be given to educating both patients and other healthcare professionals to follow the recommended policy stringently. This unit has recently started giving patients a credit card-sized information sheet that specifies the recommended duration of aspirin and clopidogrel therapy.
Finally, while the spectre of ST continues to be of some concern in relation to DES in particular, further research should focus on identifying the susceptibility of the individual to this event. Specifically, a readily available, point of care test that can accurately assess the patient’s response to antiplatelet therapy would be of considerable value, both in an attempt to predict susceptibility to ST and to modify future risk in patients who experience this complication. Tests such as modified thromboelastography show some promise in this regard.13
In conclusion, this study demonstrates that ST in a predominantly DES population occurs ‘late’ or ‘very late’ in the majority of cases and is associated with relatively long stented segments and cessation of dual antiplatelet therapy. It highlights the universal difficulty in capturing cases from a large population in a regional cardiac centre. These data suggest: (a) a need for a centralised reporting registry for ST, available to all cardiology related healthcare workers, in order to identify ST cases as completely as possible; (b) a requirement for dual antiplatelet therapy for at least a year and (c) improved education for patients and healthcare staff looking after DES patients about the importance of adhering to this policy.
Conflict of interest
This study was supported, in part, by an unrestricted educational grant from Boston Scientific Corporation. The authors have no conflict of interest to declare.
Key messages
- Stent thrombosis is associated with the discontinuation of antiplatelet therapy
- This study shows this risk appears to extend beyond six months
- This supports the administration of dual antiplatelet therapy for
at least one year post-PCI - Patients and healthcare staff need to be educated about the importance of maintaining antiplatelet therapy
- A central UK registry for stent thrombosis would be valuable
References
- Colombo A, Grube E, Koglin J et al. Early and late stent thrombosis after paclitaxel-eluting stents: analysis from the integrated TAXUS randomised trial program. J Am Coll Cardiol 2006;47(suppl A):221A.
- Rodriguez A, Mieres J, Fernandez-Pereira C, Vigo C. Coronary stent thrombosis in the current drug-eluting stent era: insights from the ERACI III trial. J Am Coll Cardiol 2006;47:205–7.
- Ong A, McFadden E, Regar E et al. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 2005;45:2088–92.
- Joner M, Finn A, Farb A et al. Pathology of drug-eluting stents in man: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202.
- Pfisterer M, Brunner-La Rocca H, Buser P et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. J Am Coll Cardiol 2006;48:2584–91.
- McFadden E, Stabile E, Regar E et al. Late stent thrombosis in drug-eluting stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519–21.
- Ferrari E, Benhamou M, Cerboni P et al. Coronary syndromes following aspirin withdrawal. A special risk for late stent thrombosis. J Am Coll Cardiol 2005;45:456–9.
- Gurbel P, Bliden K, Samara W et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis. Results from the CREST STUDY. J Am Coll Cardiol 2005;46:1827–32.
- Moreno R, Fernandez C, Hernandez R et al. Drug-eluting stent thrombosis. Results from a pooled analysis including 10 randomised studies. J Am Coll Cardiol 2005;45:954–9.
- Farb A, Burke A, Kolodgie F et al. Pathological mechanisms of fatal late
coronary stent thrombosis in humans. Circulation 2003;108:1701–6. - Joner M, Finn A, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202.
- Kotani J, Awata M, Nanto S et al. Incomplete neointimal coverage of sirolimus-eluting stents. J Am Coll Cardiol 2006;47:2108-11.
- Swallow RA, Agarwala RA, Dawkins KD, Curzen NP. Thromboelastography: potential bedside tool to assess the effects of antiplatelet therapy? Platelets 2006;17:385–92.
