Nicotinic acid (for treatment of low high-density lipoprotein [HDL]) combined with a statin has been shown previously to improve cardiovascular outcomes. To evaluate tolerability and safety of prolonged-release nicotinic acid added to statin therapy in patients at high cardiovascular risk, the Niaspan®-induced HDL-Elevation for Optimizing Risk Control (NEMO) study focused on 179 patients with atherogenic dyslipidaemia treated under usual-care conditions. Flushing was the most common treatment-related adverse drug reaction (ADR) in Ireland (32% flushed during the first month), followed by cutaneous (6.2%) and gastrointestinal (3.9%) ADRs. Mean HDL-cholesterol increased by 20%. Half of the patients elected to continue treatment after the study. In conclusion, the tolerability and safety of prolonged-release nicotinic acid was similar in Ireland compared with the overall NEMO population. It is important to take practical measures to optimise patient compliance to minimise overall cardiovascular risk.
Introduction
Cardiovascular events remain the leading cause of morbidity and mortality in developed countries, and the treatment of dyslipidaemia is central to the overall management of cardiovascular risk.1,2Although correction of hypercholesterolaemia remains the principal target for correction of the lipid profile, dyslipidaemia is heterogeneous in presentation, with many patients presenting with low high-density lipoprotein-cholesterol (HDL-C) in addition to elevated concentrations of ApoB-containing lipoproteins. A survey carried out in 11 European countries identified low HDL-C (<1.03 mmol/L in men and <1.29 mmol/L in women) in about one-third of patients undergoing treatment for dyslipidaemia.3
Moreover, limiting pharmacologic intervention to statin monotherapy addresses less than half of the overall cardiovascular risk present prior to treatment.4 Numerous epidemiological analyses have identified low HDL-C as an independent risk factor for adverse cardiovascular outcomes.5-9 Randomised intervention trials with agents that increase levels of HDL-C (reviewed elsewhere)10,11have demonstrated an improved cardiovascular outcome, in a manner consistent with these observations. Nicotinic acid (niacin in some countries) is the most effective agent currently available that increases HDL-C with increases of up to 25% commonly observed.12 Therefore, adding a nicotinic acid derivative to statin therapy is a rational strategy for optimising the lipid profile in patients with hypercholesterolaemia and low HDL-C.13,14 Such combinations have been shown to deliver improved cardiovascular outcomes relative to placebo,15 and a reduced rate of progression of atherosclerosis relative to statin monotherapy.16,17
A prolonged-release formulation of nicotinic acid (Niaspan®) is as effective in correcting lipid abnormalities as the standard, immediate-release version but causes less flushing, an important barrier to the successful therapeutic application of nicotinic acid.12 The Niaspan®-induced HDL-Elevation for Optimizing Risk Control (NEMO) study evaluated the safety and tolerability of prolonged-release nicotinic acid given under usual-care conditions in four European countries.18 Survey data have demonstrated that regional differences exist in the prevalence and severity of cardiometabolic risk factors, including the prevalence of low HDL-C.3 Accordingly, the participants in the NEMO study have been stratified according to their countries of origin, and the results of the study will be described separately for the countries containing the most substantial numbers of patients. Here, we report an analysis from the NEMO study based on patients treated in Ireland.
Methods
The methodology of the NEMO study has been published in detail,18 and only a brief account will be given here. NEMO was an observational study and all treatments were given as part of the usual-care practice of investigators. In particular, no constraints on the administration of prolonged-release nicotinic acid were applied by the study sponsor, beyond a recommendation that this treatment should be prescribed according to its label, which includes advice on stepwise titration to minimise flushing.19
Patients were eligible for the study if, at baseline, they were aged ≥18 years; had started treatment with prolonged-release nicotinic acid within the previous two months added to existing statin treatment; had HDL-C <1.3 mmol/L and/or triglycerides >1.7 mmol/L before administration of prolonged-release nicotinic acid; and were at elevated cardiovascular risk due to one or more of: (a) coronary heart disease with at least one previous myocardial infarction or stroke, (b) prior revascularisation of a coronary stenosis of at least 70% documented by angiography, (c) type 2 diabetes, or (d) Prospective Cardiovascular Münster Study (PROCAM) score indicating 10-year risk of myocardial infarction >20%.
The main objective of the study was to measure the incidence of treatment-related adverse drug reactions (ADRs), which were ADRs where the relationship of the ADR to prolonged-release nicotinic acid treatment was classified as ‘possible’, ‘probable’, ‘not assessable’, or ‘missing’. MedDRA definitions were used for ADRs. Lipid parameters and the PROCAM risk score were collected as secondary end points.
The study was conducted in accordance with relevant legislation. International conventions on the conduct of clinical studies, such as the Declaration of Helsinki, were upheld as far as applicable. The processing and handling of patient observational data was compliant with European Union Directive 5/46/EC on the processing of personal data and on the free movement of such data (24 October 1995).
Data were analysed using descriptive statistics without significance testing.
Results
Patients
Of the 1,053 patients in the main analysis of the NEMO study, 179 were recruited in Ireland. Data on the proportion of patients who elected to continue on prolonged-release nicotinic acid beyond the study period were available for 136 patients: of these, exactly half (68 patients) elected to continue on the treatment. Main reasons for not continuing after the end of the observation period were patient request (32 patients, 18% of patients recruited in Ireland), flushing (30 patients, 17%), ADRs unrelated to flushing (15 patients, 8%), and lost to follow-up (10 patients, 7%).

Demographic and disease characteristics of patients recruited are compared with those of the overall NEMO population in table 1. The proportion of female patients among patients in Ireland was higher than in the general population, although the mean age and weight of the Irish sub-population were similar to those of the overall population. Patients recruited in Ireland also had a lower prevalence of several manifestations of cardiovascular disease compared with the overall population, including coronary heart disease, myocardial infarction and cerebrovascular disease. A lower prevalence of the metabolic syndrome, family history of cardiovascular disease or smoking in Ireland may have explained these differences to some extent. Lipid profiles were similar for the two populations, except for a lower average triglyceride level in Ireland that was consistent with the lower prevalence of the metabolic syndrome in that country. Mean HDL-C at baseline was low and similar for the two populations, consistent with the inclusion criteria of the study.
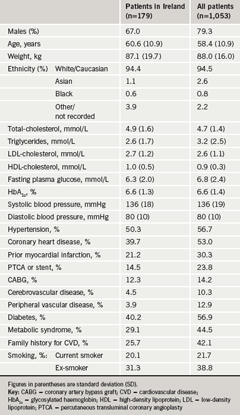
Patients required treatment with a statin to be eligible for the study. The most common statins (n= number of patients [% patients] in Ireland) prescribed were atorvastatin (n=82 [46%]), pravastatin (n=35 [20%]), simvastatin (n=14 [8%]) and rosuvastatin (n=10 [6%]), with 1–2 patients receiving other statins. The nature of the prescribed statin was not provided for 20%.
Tolerability and safety
Flushing was the most common ADR, as expected. The proportion of patients flushing during each month of the study is shown in figure 1 for patients recruited in Ireland and for the overall NEMO population. The largest proportion reporting flushing was in the first month of the study, when 32% of patients in Ireland reported flushing, declining to 17% by month six. Most episodes of flushing were rated as mild or moderate in severity by investigators. In general, the frequency and severity of flushing was similar for the population in Ireland and in the overall population. Two patients in Ireland reported flushing as a serious ADR (according to standard definitions for reporting adverse events in clinical trials). Both patients recovered after withdrawal of medication.
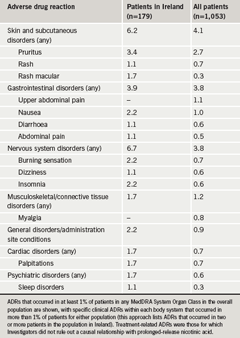
The frequency of gastrointestinal ADRs was similar for the two populations studied, although patients in Ireland reported a slightly higher incidence of skin and subcutaneous disorders and nervous system disorders (table 2). Four patients recruited in Ireland (2%) reported a total of seven treatment-related serious ADRs other than flushing (compared with 1% of patients in the overall NEMO population).
Physicians rated overall tolerability at study end for 122 of the patients (68%) recruited in Ireland. For these patients, the overall tolerability of prolonged-release nicotinic acid was rated as ‘very good’ for 12.3% (vs. 17.7% for the overall population), ‘good’ for 32.8% (vs. 35.7%), and ‘acceptable’ for 18.0% (vs. 19.6%).
Efficacy measurements
The principal effects of prolonged-release nicotinic acid on lipid parameters were as expected, with marked increases in HDL-C and decreases in triglycerides (figure 2). A larger decrease in low-density lipoprotein-cholesterol was observed in patients in Ireland compared with the overall population (figure 2).
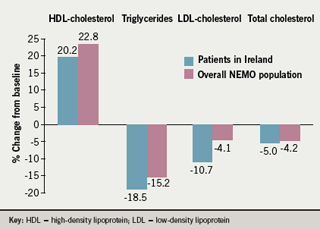
Physicians rated efficacy at study end for 118 of the patients (66%) recruited in Ireland. The overall efficacy of prolonged-release nicotinic acid was rated as ‘very good’ for 9.3% (vs. 13.1% for the overall population), ‘good’ for 28.0% (vs. 33.4%), and ‘acceptable’ for 25.4% (vs. 26.2%). Mean PROCAM score (10-year risk of myocardial infarction or coronary death) fell by 3.5 (standard error of the mean [SEM] 0.7) from a baseline value of 44.9, a change similar to the decrease of 4.0 (SEM 0.3) from a baseline value of 45.6 in the overall population.
Discussion
The NEMO study provided an evaluation of the tolerability and safety of prolonged-release nicotinic acid added to statin therapy in four European countries. Although this study lacked a placebo or other control, the study was carried out under usual-care conditions, and its results are therefore directly applicable to routine clinical practice. In general, the tolerability and safety profiles of prolonged-release nicotinic acid were as expected from previous clinical experience, with flushing the principal tolerability issue, and gastrointestinal and cutaneous side effects the most common ADRs other than flushing.12 The frequency of serious ADRs was low with no evidence of myopathy or hepatic dysfunction.
Although the effects of the study treatment were generally similar in Ireland versus the overall population, some interesting differences between these populations emerged. For example, the prevalence of several manifestations of cardiovascular disease was lower at baseline in the Ireland cohort relative to the overall study population. The reason for this is unclear. The lower proportion of males treated in Ireland relative to elsewhere may have contributed to this observation. Another possibility is that, as the prescription of treatments in this non-interventional study was directed entirely by the usual practice of physicians, it may be that lipid-modifying treatments other than statins are more readily prescribed in the absence of overt cardiovascular disease in Ireland relative to the other countries (Austria, the Netherlands and Sweden). However, there was little difference in overall cardiovascular risk between the populations described here in terms of their PROCAM score at baseline.
Another difference emerged from the rating of tolerability and efficacy by investigators. Physicians in Ireland gave lower ratings for overall tolerability than investigators in other countries, despite a similar incidence of flushing and other ADRs. Similarly, the rating of overall efficacy was lower in Ireland, despite similar effects on HDL-C, and an apparently greater effect on low-density lipoprotein cholesterol. Whether these differences resulted from differing expectations of physicians in Ireland with reference to the expected therapeutic profiles of treatments, or from some other factor, is unclear. It should be noted however, that the substantial proportion of patients for whom overall ratings of efficacy and tolerability were unavailable represents a limitation of this study.
Half of the patients for whom data were available elected to continue treatment with prolonged-release nicotinic acid after the study. Correction of low HDL-C remains an important therapeutic objective for the overall management of cardiovascular risk. Nicotinic acid remains the most effective treatment currently available for raising HDL-C (it should be noted here that the failure of the prototype cholesteryl ester transfer protein inhibitor, torcetrapib, to reduce cardiovascular risk20,21 was likely due to other effects rather than its effects on HDL-C).22,23 This study highlights the practical issue of optimising compliance with this regimen. In particular, patients should be educated on the strong likelihood that they will experience flushing, and briefed on practical measures to minimise its frequency and impact.24 These include avoiding alcohol or spicy foods (which themselves promote flushing) at the time of ingestion of nicotinic acid. The Prescribing Information for this treatment recommends ingestion of nicotinic acid at bedtime. This is a pragmatic measure to minimise the impact of flushing on daily activities, especially as patients will often sleep through a mild flushing episode.19 In addition, it has been known for more than 25 years that analgesic doses of non-steroidal anti-inflammatory agents will prevent or terminate a flushing episode,25 although lower doses in the range used for cardiovascular prophylaxis are less effective.26
Conclusion
A combination of prolonged-release nicotinic acid added to a statin was safe and well tolerated, and was associated with additional benefits to lipid profile compared with statin monotherapy. The increase in HDL-C induced by prolonged-release nicotinic acid may lead to a reduction in cardiovascular risk beyond that provided by low-density lipoprotein-cholesterol reduction alone.
Conflict of interest
The NEMO study was funded by an educational grant from Merck Serono. JK has acted as a consultant to Merck Serono, and has delivered lectures at sponsored symposia. MO’R was an investigator in the NEMO Study. UH is an employee of Merck Serono.
Key messages
- The tolerability and safety of prolonged-release nicotinic acid were evaluated under usual-care conditions in patients with atherogenic dyslipidaemia in Ireland
- Flushing was the most common treatment-related adverse drug reaction (ADR; 32% during the first month), followed by cutaneous (6.2%) and gastrointestinal (3.9%) ADRs
- Prolonged-release nicotinic acid increased mean high-density lipoprotein (HDL)-cholesterol by 20%
References
- Task force on diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes and cardiovascular diseases: full text. Available from: http://www.escardio.org [accessed April 2008].
- De Backer G, Ambrosioni E, Borch-Johnsen E et al. Cardiovascular disease prevention in clinical practice (European guidelines on). Available from: http://www.escardio.org [accessed April 2008].
- Bruckert E, Baccara-Dinet M, McCoy F, Chapman J. High prevalence of low HDL-cholesterol in a pan-European survey of 8545 dyslipidaemic patients. Curr Med Res Opin 2005;21:1927–34.
- Baigent C, Keech A, Kearney PM et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins.Lancet 2005;366:1267–78.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med1977;62:707–14.
- Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124(suppl):S11–S20.
- Gordon DJ, Probstfield JL, Garrison RJ. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8–15.
- Turner RC, Millns H, Neil HA et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ1998;316:823–8.
- Chapman J. Beyond LDL-cholesterol reduction: the way ahead in managing dyslipidaemia. Eur Heart J 2005;7(suppl):F56–F62.
- Brown BG. Maximizing coronary disease risk reduction using nicotinic acid combined with LDL-lowering therapy. Eur Heart J 2005;7(suppl):F34–F40.
- Drexel H. Reducing risk by raising HDL-cholesterol: the evidence. Eur Heart J 2006;8(suppl):F23–F29.
- Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E. Prolonged-release nicotinic acid for the management of dyslipidemia: an update including results from the NAUTILUS study. Vasc Health Risk Manag 2007;3:467–79.
- Chapman MJ, Assmann G, Fruchart JC, Shepherd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid–a position paper developed by the European Consensus Panel on HDL-C. Curr Med Res Opin 2004;20:1253–68.
- Gotto AM Jr, Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol2004;43:717–24.
- Brown BG, Zhao X-Q, Chait A et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583–92.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004;110:3512–17.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin2006;22:2243–50.
- Birjmohun RS, Kastelein JJP, Poldermans D, Stroes ESG, Hostalek U, Assmann G. Safety and tolerability of prolonged-release nicotinic acid in statin-treated patients. Curr Med Res Opin2007;23:1707–13.
- Niaspan® prescribing Information, Merck Serono, Darmstadt, Germany.
- Nissen SE, Tardif JC, Nicholls SJ et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304–16.
- Kastelein JJ, van Leuven SI, Burgess L et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620–30.
- Yvan-Charvet L, Matsuura F, Wang N et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol 2007;27:1132–8.
- Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol 2007;27:257–60.
- Reasner CA. Achieving the therapeutic benefits of Niaspan® in daily practice. Eur Heart J 2006;8(suppl):F68–F73.
- Wilkin JK, Wilkin O, Kapp R, Donachie R, Chernosky ME, Buckner J. Aspirin blocks nicotinic acid-induced flushing. Clin Pharmacol Ther 1982;31:478–82.
- Whelan AM, Price SO, Fowler SF, Hainer BL. The effect of aspirin on niacin-induced cutaneous reactions. J Fam Pract 1992;34:165–8.
