Major new trials reported at the American Heart Association 2008 Scientific Sessions, held in New Orleans on November 8th – 12th, showed encouraging results for rosuvastatin in the primary prevention of cardiovascular events, while exercise in heart failure patients produced modest health benefits.
JUPITER shows large cardiovascular risk reduction in primary prevention
The eagerly awaited landmark JUPITER trial shows that the treatment of apparently healthy patients – who had low levels of low-density lipoprotein (LDL) cholesterol but elevated C-reactive-protein (CRP) levels – with rosuvastatin cuts their risk of cardiovascular disease morbidity and mortality by around 50%.
The results were the first late-breaking trial data reported here at the AHA 2008 Scientific Sessions and were also published in the New England Journal of Medicine (N Engl J Med 2008; 359: 2195-207).
JUPITER was designed as a four-year study but was stopped in March this year after just 1.9 years, based on recommendations from an independent data monitoring board and the JUPITER steering committee, after the company reported unequivocal evidence of a reduction in cardiovascular morbidity and mortality among patients treated with rosuvastatin compared with those on placebo.
Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) was presented at AHA by Dr Paul Ridker (Brigham and Women’s Hospital, Boston, MA, USA), who said that the benefits of the statin extended to all subgroups. This included “robust reductions in cardiovascular events with statin therapy in women and black and Hispanic populations, for which data on primary prevention are limited”.
The findings led to much debate about whether CRP testing should become part of primary prevention guidelines. In an editorial accompanying the published study (N Engl J Med 2008; 359: 2280-2), Dr Mark Hlatky (Stanford University School of Medicine, CA, US) says: “Guidelines for primary prevention will surely be reassessed on the basis of the JUPITER results,” altough he is cautious about how much of an impact the findings will have on clinical practice.
Results
JUPITER was designed to assess whether statins should be given to apparently healthy individuals with normal LDL cholesterol levels (< 3.4 mmol/L) but elevated CRP (> 2.0 mg/L, and 17,802 healthy men (aged > 50 years) and women (aged > 60 years) were randomised to rosuvastatin 20 mg or placebo.
Among patients treated with rosuvastatin, LDL cholesterol levels were cut in half, decreasing from a median 108 mg/dL (2.8 mmol/L) at baseline to 55 mg/dL (1.4 mmol/L) at 12 months. CRP levels were also significantly reduced, declining from 4.2 mg/L at baseline to 2.2 mg/L at 12 months. And triglyceride levels were reduced 17% from baseline among those treated with statin therapy. These effects persisted over the course of the study.
After 1.9 years of follow-up, rosuvastatin 20 mg significantly reduced the primary end point – a composite of non-fatal myocardial infarction (MI), non-fatal stroke, hospitalisation for unstable angina, revascularisation, and confirmed death from cardiovascular causes – by 44% compared with individuals treated with placebo.
This reduction was observed among nearly all of the individual end points, including a 55% reduction in non-fatal MI, a 48% reduction in the risk of non-fatal stroke, and a 47% reduction in the risk of hard cardiac events (a composite of MI, stroke, and death from cardiovascular causes).
In terms of absolute benefits, the proportion of patients who had an MI, stroke, revascularisation, or hospitalisation for unstable angina or died from cardiovascular causes was 1.6% in the rosuvastatin arm and 2.8% in the placebo arm, an absolute risk reduction of 1.2%. Similarly, the proportion of patients with hard cardiac events – cardiovascular death, MI, and stroke – was reduced from 1.8% in the placebo arm to 0.9% in the rosuvastatin arm, an absolute reduction of 0.9%.
In terms of side effects, the rosuvastatin group did not have a significant increase in myopathy or cancer but there was a higher incidence of physician-reported diabetes, and they also had significantly higher glycated haemoglobin levels.
In his editorial, Dr Hlatky calculated that 120 patients need to be treated for 1.9 years to prevent one death from cardiovascular causes, MI, or stroke, and this benefit needs to be balanced against concerns about significantly higher glycated haemoglobin levels and increased diabetes incidence seen in the rosuvastatin arm. The JUPITER investigators, on the other hand, estimate the number needed to treat (NNT) with rosuvastatin for two years to prevent one primary end point is 95 and just 31 need to be treated for four years to prevent one primary-end-point event.
Debate
Observers pointed out that, as statins lower both LDL and CRP, it cannot be determined whether cholesterol lowering, a reduction in inflammation, or a combination of both is responsible for the reductions in risk seen.
Many experts said the current LDL cholesterol thresholds for lipid-lowering therapy are arbitrary, and a poor indicator of cardiovascular risk, because many patients with heart attacks have normal LDL cholesterol values. The current LDL cholesterol cut-off level of 100 mg/dL (2.6 mmol/L) in the US is still too high if a patient has other markers of risk, such as increased age, obesity, and hypertension, doctors commented. And some pointed out that those in JUPITER were not necessarily without risk as the average body mass index (BMI) in the study was 28.3 kg/m2.
In his editorial, Dr Hlatky said the design of JUPITER provides only limited information about the role of CRP testing in clinical practice, since investigators did not compare subjects with and without CRP measurements, nor did they compare the use of CRP with use of other markers of cardiovascular risk.
Experts said more widespread screening of CRP values needs more investigation. The test has high variability and CRP is elevated with infections and injuries, so abnormally high CRP levels do not always reflect arterial injury or cardiovascular disease risk.
Many in the US use CRP as a “tie-breaker” to make decisions about which men over 50 years and women over 60 will benefit from statins. Explaining and implementing this may be easier for physicians and patients than trying to explain why ‘borderline’ high blood pressure and being ‘a little overweight’ are bad, some commented.
The study has been welcomed by UK physicians. Dr Terry McCormack, Former Chairman of the Primary Care Cardiovascular Society, and a GP in Whitby, said: “JUPITER is as important as the ground breaking Scandinavian Simvastatin Survival Study (4S) in terms of proving the value of statins, this time in primary prevention, and will be the basis for future debate and a re-examination of the guidelines.”
Cost is likely to be an issue. Dr McCormack estimates the UK cost of using rosuvastatin to prevent one event based on the NNT of 95 would be roughly £73,000 over two years, not including the cost of CRP screening or the amount saved by avoiding the event.
SEARCH shows increased lipid lowering but more myopathy with high-dose simvastatin
Intensive versus less intensive low-density lipoprotein (LDL) cholesterol lowering, comparing 80 mg and 20 mg doses of simvastatin, showed the 80 mg dose failed to reduce vascular events significantly, although there was a reduction in LDL with this higher dose.
The results are consistent with previous trials of more versus less statin, said the investigators of the SEARCH study, which also looked at lowering homocysteine using folic acid and vitamin B12. This did not have any effect on vascular events compared with placebo.
Dr Rory Collins (Clinical Trial Services Unit, Oxford) presented SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) results during a late-breaking clinical trial session. The study was carried out in more than 12,000 myocardial infarction (MI) survivors and his colleague Dr Jane Armitage (Clinical Trial Services Unit) discussed the results at a press conference. She said those who received the 80 mg dose of simvastatin had a 0.35 mmol/L (14 mg/dL) lower LDL than those who got the 20 mg dose, which translated into a 6% further reduction in vascular events, “which on its own was not significant but is in line with all the other combined more-versus-less data. When we look at the combined data, we see a very clear picture emerging”.
But discussant of the SEARCH study, Dr Peter Wilson (Emory University, Atlanta, GA, USA), said: “This was a null trial that was morphed into a positive meta-analysis. I don’t want to be unfair, but I think that’s what we just saw.”
More myopathy
Also, there were significantly more cases of myopathy with the 80 mg dose of simvastatin than with the 20 mg dose (53 vs. 3), Dr Armitage noted, adding that “quite clearly the myopathy risk is higher with 80 mg and safety is an issue with simvastatin”. Other doctors at the meeting said they would likely choose a more potent statin than simvastatin if they needed to intensively lower LDL cholesterol.
LDL results
In SEARCH, 12,064 MI survivors were randomised to either 20 mg or 80 mg of simvastatin daily for the more versus less LDL-lowering comparison and to folic acid 2 mg plus vitamin B12 1 mg daily or placebo for the homocysteine-lowering comparison, for a total of seven years.
The primary end point was major vascular events, consisting of major coronary events, stroke, and revascularisation. There was no difference in the primary end point between the groups: 1,477 events (24.5%) in the 80 mg simvastatin group vs. 1,553 events (25.7%) in the lower-dose group (p=0.10).
Reassuringly, however, there was no evidence that further reducing LDL increased the risk of cancer, which has previously been a concern, Dr Collins noted.
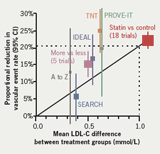
Although not significant, these findings are consistent with other trials in the field, including TNT, PROVE-IT, IDEAL, and A to Z, said Dr Armitage, and show that larger reductions in LDL cholesterol with intensive statin therapy produce further reductions in each of the components of major vascular events, including revascularisation and non-fatal MI/coronary death. The combined evidence illustrates that further reducing LDL by 0.5 mmol/L provides an additional 16% relative reduction in vascular events, a 1.5 mmol/L decline provides a 33% relative risk reduction, and a 2 mmol/L drop gives a further 40% relative reduction in events, she concluded (figure 1).
Discussant, Dr Wilson, said the difference seen between the two statin groups in the SEARCH trial “was not enough to show that lower is better, and the LDL was only modestly lower in the 80 mg simvastatin group,” although he commented that a difference may have emerged had the trial lasted longer. “Does lower LDL lead to lower cardiovascular risk post-MI? The SEARCH trial didn’t answer that question,” he said.
Homocysteine results
In the homocysteine-lowering arm of SEARCH, there were 1,537 major vascular events in the folic acid/vitamin B12 arm versus 1,493 in the placebo arm, to give a risk ratio of 1.04. Dr Armitage said there is “clear evidence from SEARCH that homocysteine lowering does not affect the risk of vascular events – it’s really a very robustly negative result, and there were no subgroups in whom there was any benefit”.
UK reaction
Back in the UK, the results have led to organisations calling for a review of the current NICE (National Institute of Health and Clinical Excellence) guidance. “Because of the relatively high incidence of myopathy in patients taking high dose simvastatin, the study suggests that aggressive cholesterol lowering may be more safely achieved using lower doses of more potent statins. Consequently the current NICE guidelines may need to be reviewed,” Professor Peter Weissberg, Medical Director of the British Heart Foundation said after the meeting.
Dr Fran Sivers, Director of the Primary Care Cardiovascular Society agreed: “Clearly there are side effect issues with high dose simvastatin in a significant number of patients,” she said. “Clinicians have long thought this to be the case and the new SEARCH data confirms our suspicions. We therefore call on NICE to review its recommendations for lipid lowering in high risk patients given the findings of this study.”
I-PRESERVE shows disappointing results with preserved left ventricular function heart failure
Patients with heart failure and preserved left ventricular ejection fraction (LVEF) did not benefit from four years of treatment with the angiotensin receptor blocker (ARB) irbesartan in I-PRESERVE.
The study which was also published simultaneously in the New England Journal of Medicine (DOI: 10.1056/NEJMoa0805450) randomised 4,128 patients aged >60 years with New York Heart Association (NYHA) class 2-4 heart failure and an LVEF >45% to receive irbesartan, initiated at 75 mg/day and titrated to the target dosage of 300 mg/day (the average dosage achieved was 275 mg/day) or placebo. There was no difference in mortality or cardiovascular events between the two groups by the end of the study.
This is the third major prospective trial to test the effects of a drug that inhibits parts of the renin-angiotensin-aldosterone system (RAAS) in patients with this common form of heart failure, delegates heard.
The results are consistent with both the CHARM-Preserved trial of the ARB candesartan and the PEP-CHF study of the ACE inhibitor perindopril in similar populations, said Dr Peter E Carson (Washington VA Medical Center, Washington, DC, USA) who presented the I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) study at the meeting.
“For this large group of patients, constituting up to half of all heart failure, there continues to be no specific evidence-based therapy,” he observed. “In order for the field to move forward, a better understanding is needed of the mechanisms underlying this syndrome and the additional potential targets for treatment.”
Discussion
Discussant Dr Margaret M Redfield (Mayo Clinic, Rochester, MN, USA) said, “I think the three trials taken together don’t provide strong support for adding these agents once the blood pressure is well controlled.” The findings are important, she added, “because ACE inhibitors and ARBs are very commonly used to treat this condition even though there are no compelling randomised clinical trials to support it”.
But panelist Professor Philip Poole-Wilson (Imperial College London) had a slightly different take on the study.
“You have here a trial with [about] 40% on ACE inhibitors, [about] 70% on beta blockers, and a quarter on spironolactone. All of those drugs interact with the RAAS system. So what this trial has not shown, in my view, is that inhibiting that system in this group of patients is not beneficial.”
“What you’ve shown is that this particular drug, in a population absolutely awash with other drugs inhibiting the RAAS system, doesn’t add any further benefit,” he told a press briefing.
Physicians Health Study shows no benefit for antioxidants
Neither vitamin E nor vitamin C protected against cardiovascular disease in a long-term study in 14,000 male doctors reported at a late-breaking clinical trial session. Use of the antioxidant vitamin supplements for eight years did not reduce a composite of non-fatal myocardial infarction (MI), non-fatal stroke, or cardiovascular death in the Physicians’ Health Study II (PHS2).
The results were also published simultaneously in the Journal of the American Medical Association (JAMA 2008; 300:2123-33).
“In conclusion, PHS demonstrated that individual vitamin E and vitamin C supplements did not reduce the risk of major cardiovascular events,” Dr J Michael Gaziano (Brigham and Women’s Hospital and the Harvard School of Public Health, Boston, MA), US, senior author of the trial, told a press conference.
He said the results add to the growing consensus about these vitamins’ lack of cardiovascular protection based on several earlier trials that failed to find any effect.
Despite uncertainty about long-term health benefits, vitamin supplements are widely used, with billions of dollars of sales each year in the US and other western nations. “More than half of Americans consume vitamin supplements on a weekly basis, and vitamin E and vitamin C are among the most widely used,” he said.
He added that these findings underline how important it is to take results from observational studies into randomised controlled trials.
Dr Barbara V Howard (MedStar Research Institute, in Hyattsville, MD, US), chair of the American Heart Association Council on Nutrition, discussant for this trial, said: “Given these results and the caveat about observational data, I think it is prudent to follow the guidelines that people need to balance their calories and activity to maintain weight, eat plenty of vegetables and fruits and whole grains, reduce their saturated and trans fat, and reduce their sodium and sugar intake. This is the way… cardiovascular disease can be reduced, and in these hard economic times, maybe we can save some money and not buy these supplements,” she concluded.
HF-ACTION: quality of life improves with exercise
Heart-failure patients participating in an exercise-training programme improved their quality of life, with the benefits appearing early and being sustained for three years, results from the HF-ACTION study show. However, the primary end point of the study – a reduction in all-cause mortality or all-cause hospitalisation – was not reached in those on the exercise program compared with usual care.
Doctors at the meeting said this was likely due to a clinical trial effect, whereby the usual-care arm exercised more than they would have if not enrolled. They insisted that the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) findings are consistent with prior clinical data, and support the use of exercise training in this population.
“The goal of clinicians treating heart failure patients is to help them feel better, to prolong life, but also to improve quality of life,” said investigator Dr Ileana Piña (Case Western Reserve University, Cleveland, OH, USA), at a press conference.
“Our results show that participation in a training programme produced modest, but significant, improvement in health status in patients with heart failure compared with usual care. Improvement was associated with exercise training, and occurred early during supervised training and was sustained. The results were consistent among subgroups.”
Study details
In HF-ACTION, 2,331 heart-failure patients (New York Heart Association class 2-4, ejection fraction <35%) were randomised to either the structured-exercise group or usual care.
In the structured group, 36 supervised training sessions were provided for 30 minutes of exercise three times per week, and halfway through this period, patients were given a treadmill or stationary bicycle to use at home along with a heart-rate monitor and were advised to work out five times per week at moderate intensity for 40 minutes. In contrast, patients in the usual care group were simply told at the study outset to exercise at moderate intensity, 30 minutes per day, but were not supervised or encouraged along the way.
The overall study findings – reported by Dr Christopher O’Connor (Duke University Medical Center, Durham, NC, US) – showed no difference in the primary end point between the two groups.
But a secondary analysis that took into account pre-specified major prognostic factors identified at the outset of the trial – heart-failure aetiology, exercise duration, left ventricular ejection fraction, Beck depression inventory, and history of atrial fibrillation/flutter – did show significant reductions in hard clinical events. When the analysis was adjusted for these prognostic factors, the composite primary end point was significantly reduced by 11%, and a composite of cardiovascular mortality/heart-failure hospitalisation was reduced by 15%.
Quality of life substudy shows early benefit
A quality of life (QOL) substudy, also presented by Dr Piña, showed an early improvement, within three months, in the health status of patients participating in the structured-exercise program, using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The benefit was sustained to three years, with the overall scores virtually unchanged from three months to 36 months. Moreover, all health-status scales were improved, including physical limitations and symptoms.
Discussant for the overall study presentation, Professor Philip Poole-Wilson (Imperial College London) said: “Some people would say, well, they missed their primary end point, let’s all go home. I think that would be very wrong.” The adjusted outcomes are “very compelling” and consistent with the findings from exercise tests in the two groups, he noted. “I think this trial does support the use of exercise and it will strengthen the guidelines.”
New ATHENA data for dronedarone
Two new post-hoc analyses of the landmark ATHENA trial were reported at the meeting last week, showing that the new anti-arrhythmic drug, dronedarone, reduced the time spent in hospital and also the incidence of first atrial fibrillation (AF) recurrence and electrical cardioversion compared with placebo.
Effect of dronedarone on hospitalisations
Patients in the ATHENA trial were randomised to either 400 mg bid of dronedarone or placebo, and the primary outcome was time to first cardiovascular hospitalisation or death from any cause. The new anti-arrhythmic drug reduced this combined end point, as first reported at the Heart Rhythm Society meeting earlier this year.
In terms of the cardiovascular hospitalisations alone, the new post-hoc analysis showed there were 675 first hospitalisations in the patients on dronedarone compared with 859 on placebo (hazard ratio 0.75; p<0.001).
The main reasons for first hospitalisations on dronedarone/placebo were AF: 296/457, ischaemic heart disease 93/102 and heart failure 78/92.
Examining total hospitalisation burden, there were 9,995 nights in hospital on dronedarone and 13,986 on placebo, a reduction of 28% with the new drug (p<0.001). For cardiovascular hospitalisations, these figures were 5,875 nights and 9,073 nights respectively, a reduction of 35% with dronedarone compared with placebo (p<0.001).
“In patients with paroxysmal or persistent AF, dronedarone substantially reduces the risk of cardiovascular hospitalisation,” concluded Dr Christian Torp-Pedersen (Gentofte University Hospital, Copenhagen, Denmark).
“The incidence of AF-related hospitalisations has dramatically increased in recent years… these new ATHENA data showed that, for the first time, an anti-arrhythmic drug significantly and consistently reduced hospitalisation incidence and duration… in this patient population,” he adds in a Sanofi-Aventis statement.
Rhythm and rate-controlling effects of dronedarone
In the second post-hoc analysis reported at the AHA, the rhythm and rate controlling properties of dronedarone in ATHENA were analysed.
The median time to first AF/atrial flutter recurrence of patients in sinus rhythm at baseline was prolonged from 498 days in the placebo group to 737 days in the dronedarone group (HR 0.75; p<0.001).
Of 586 placebo patients with AF/atrial flutter at randomisation, 195 underwent cardioversion, compared with 151 of 569 dronedarone patients (hr 0.76; p=0.01).
And fewer patients in the dronedarone group developed permanent AF during the study: 178 (7.7%) versus 295 (12.7%) in the placebo arm (p<0.001), and dronedarone decreased mean heart rate during AF to 78 bpm compared with 87 bpm for placebo (p<0.001).
Dronedarone exhibits both rhythm and rate-controlling properties in AF/atrial flutter patients and these effects likely contribute to the reduction of important clinical outcomes observed in ATHENA, concluded Dr Richard L Page (University of Washington, Seattle, USA).
“It is intriguing that there was a trend toward reduction of the primary end point of cardiovascular hospitalisation or death even in patients with permanent AF, suggesting that the benefit of dronedarone may not only be linked to arrhythmia control,” he adds in a Sanofi-Aventis press release.
