National guidance recommends specific durations for clopidogrel and aspirin dual therapy post ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) and following percutaneous coronary intervention (PCI). Primary care clinicians highlighted that patients were frequently discharged from acute trusts without any communication as to the indication for or intended duration of clopidogrel therapy. An initial audit across four of the six acute trusts demonstrated significant variation in the use of clopidogrel and aspirin dual therapy across the sector, with 26% of discharge prescriptions giving no clear indication for therapy and 30% of discharge prescriptions giving no indication of the intended duration of therapy.
The South East London Cardiac and Stroke Network (SELCSN) cardiac prescribing forum, with input from consultant cardiologists, GPs, pharmacists and others, developed and implemented a consensus guideline for the prescribing of clopidogrel across the sector. In addition, it was agreed that acute trust pharmacy departments would not dispense clopidogrel for discharge unless the duration was clearly documented on the discharge prescription.
Re-audit demonstrated an increase in the proportion of discharge prescriptions with an indication for and duration of clopidogrel therapy: 92.5% and 85%, respectively. Failure to implement the SELCSN guidance in one trust had a significant impact on the overall results; however, four of the six trusts managed to achieve over 95% compliance in terms of communicating a clear indication for and duration of clopidogrel therapy. In addition, the number of different regimens in use was lower during the re-audit period indicating a move to more consistent prescribing of clopidogrel across the sector, although there remains significant variation between trusts.
Next steps for the SELCSN are to address clopidogrel prescribing in the trust with poor outcomes in the audit to ensure full implementation of the local guidance, development of a tool to audit prescribing of clopidogrel in primary care to assess appropriate cessation of dual therapy and re-audit of acute trust discharge communication to ensure the good practice which has been implemented is maintained in the long term.
Introduction
Over the past five years, clopidogrel, often in combination with aspirin, has become standard therapy for patients with a number of cardiovascular disorders. Dual therapy with aspirin and clopidogrel is particularly important in the management of ST-elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) and following intra-coronary stent placement. Evidence from clinical trials has demonstrated that the benefits of dual therapy are greatest where aspirin and clopidogrel are initiated early following an acute event or prior to a percutaneous coronary intervention (PCI). The optimal duration of clopidogrel post event or intervention depends on the indication for which it has been prescribed.
Current guidance issued by the National Institute for Health and Clinical Excellence (NICE) recommends that patients should be prescribed aspirin and clopidogrel dual therapy for:
- 12 months post NSTEMI1,2
- At least four weeks post STEMI.3
In addition, the British Cardiovascular Intervention Society (BCIS) recommends that after a PCI, aspirin/clopidogrel dual therapy is continued for one month following elective bare metal stent insertion and for 12 months following insertion of a drug-eluting stent.4
Limited durations of clopidogrel/aspirin dual therapy are recommended as the combination is associated with an increased risk of bleeding. In addition, the prescribing of clopidogrel is associated with significant expenditure for local primary care trusts (PCTs), which has been increasing year on year. Ensuring clopidogrel is appropriately discontinued at the end of the required dual therapy course is therefore important to limit bleeding risks and contain drug costs.
It is within this context that the South East London (SEL) cardiac prescribing forum was tasked with improving clopidogrel prescribing across the sector. One of the first issues identified by the group was the inconsistency in communication of clopidogrel indication and duration at discharge from acute care. Clearly, without this information it is impossible for primary care to know at what point clopidogrel therapy can safely be discontinued. In addition, anecdotally there were significant differences in practice across the two tertiary centres and four district general hospitals within the sector. As a result there was little clarity regarding the use of dual aspirin and clopidogrel across the six PCTs.
Methods
Auditing discharge communication
The first step in tackling the issues raised was to undertake an audit of clopidogrel prescribing at discharge from acute care to quantify the problem. Pharmacists within each acute centre were asked to retrospectively analyse all discharge prescriptions (known as ‘TTAs’) for clopidogrel issued for cardiac patients over a period of one month. Data were received for a total of 249 patients discharged from four of the six acute trusts during January 2007.
This preliminary audit identified that:
- the indication for clopidogrel was absent or unclear on 26% of discharge prescriptions
- the duration of clopidogrel was absent or unclear on 30% of discharge prescriptions
- communication of a clear clopidogrel duration ranged from 30% to 96%, depending on the originating trust
- where a duration was stated, this had been added or amended by the pharmacist in 28% of cases.
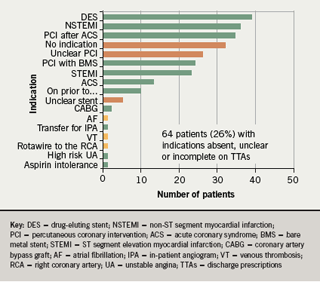
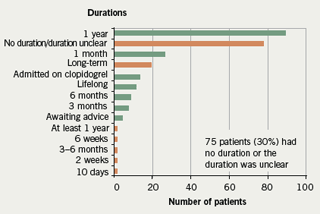
In addition, it was noted that there were 17 different indications for dual aspirin/clopidogrel therapy given (figure 1) and 14 different durations recommended (figure 2) during the course of the one month audit.
Objectives for the cardiac prescribing forum
As a result of this audit, two key objectives for the cardiac prescribing forum were established:
- To achieve consensus around indications for and durations of clopidogrel therapy across all primary and secondary care organisations within the sector.
- To improve the communication of this information at discharge from secondary care to primary care.
Developing and implementing consensus guidelines
A one-page key messages document summarising the appropriate use of clopidogrel in combination with aspirin for cardiac patients was developed with input from interventional cardiologists, general practitioners, specialist cardiac pharmacists and prescribing advisers. This document lists the key indications for which clopidogrel and aspirin should be prescribed, and the recommended duration of clopidogrel therapy for each specific indication. Other elements of good practice in clopidogrel prescribing were also emphasised, such as the place of clopidogrel monotherapy, the importance of not stopping clopidogrel early and the use of patient-held clopidogrel cards to improve communication and concordance (box 1).
The key messages document was supported by a ‘Frequently Asked Questions’ (FAQ) document that was drawn up by the pharmacists on the cardiac prescribing forum. This summarised the evidence that underpinned the key messages, in order to assist clinicians in understanding and implementing them in clinical practice. The FAQ can be found at: http://www.selcardiacnetwork.nhs.uk/files/ClopKMandFAQcombinedSELCNagreed Sept07_notels.pdf.
Once endorsed by the SELCSN prescribing forum, the key messages and FAQ were taken through the appropriate ratification processes in each of the local PCTs and acute trusts. Local implementation was led by the pharmacy representatives on the cardiac prescribing forum throughout June to August 2007.
Improving communication
The baseline audit established the need to improve the communication of the intended clopidogrel duration at discharge from acute care. The key messages document emphasised the need for a clear indication and duration of clopidogrel therapy to be communicated to primary care at discharge. One acute trust had already implemented a protocol, whereby the pharmacy department would not dispense clopidogrel for discharge unless a clear indication and duration was evident on the discharge summary. This proved very successful in ensuring the required information was available to primary care, to the extent that 96% of all discharge prescriptions issued from that acute trust during the audit month included the appropriate data on duration. This practice was endorsed by the SELCSN prescribing forum, and all acute trust pharmacy departments were requested to implement a similar protocol.
Results
Re-auditing discharge communication
Following the implementation phase and six months after the initial audit, a re-audit of clopidogrel prescribing at discharge from cardiac units in secondary/tertiary care was undertaken. All discharge prescriptions issued from cardiology during September 2007 were identified retrospectively and audited by the pharmacists in each acute trust. Data were received for 311 patients discharged from all six of the acute trusts during the audit month.
- The follow-up audit demonstrated an improvement in the communication of the indication for and intended duration of clopidogrel therapy at discharge compared with the baseline audit.
- The indication for clopidogrel was absent or unclear on 9.4% of discharge prescriptions (compared to 26% in January 07)
- The duration of clopidogrel was absent or unclear on 15% of all discharge prescriptions (compared to 30% in January 2007)
- Communication of a clear clopidogrel duration ranged from 5% in the worst performing trust to 100% in the best performing trusts (n=2)
- Where a duration was stated, this had been added or amended by the pharmacist in 19.7% of cases.
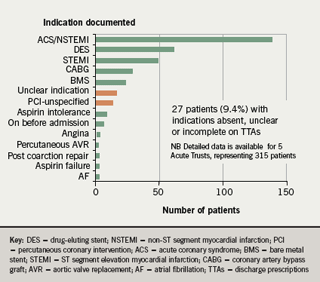
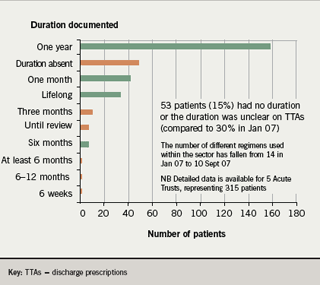
In addition, in the re-audit, the number of different indications given for clopidogrel use fell from 17 in January 2007 to 14 in September 2007 (figure 3); and the number of different durations recommended fell from 14 to 10 (figure 4).
Discussion
The development and implementation of a consensus guideline for clopidogrel prescribing combined with a system to improve communication of indication for and duration of clopidogrel/aspirin dual therapy at discharge from acute trusts has improved clopidogrel prescribing. The number of different indications and durations cited at discharge across the sector has fallen, although still far exceeds the number of approved indications and durations listed in the key messages document. This demonstrates a move towards better consistency in clopidogrel prescribing across the sector but also highlights the need for ongoing work.
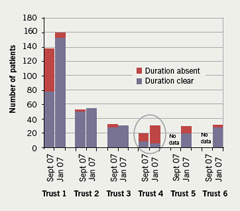
The overall results, however, mask the degree of compliance with the SELCSN consensus guidelines by the individual trusts within the sector and the significant improvements in communication seen in some of the acute trusts. For example, for the communication of duration at discharge a breakdown of the data by trust demonstrates good levels of compliance in four of the six trusts. A significant improvement in communication of duration is seen in Trust 1 (from 58.7% in Jan 07 to 95.8% in Sept 07) and in Trust 3 (from 87.8 to 100%). Trust 2 already had and maintained good levels of compliance (96% in Jan 07; 100% in Sept 07), while comparative data were not available for Trusts 5 and 6 from the baseline audit (figure 5). Trust 4 can be seen to account for the majority of the discharge prescriptions with missing durations during the re-audit period, only 30% of discharge prescriptions for clopidogrel from this trust stated a duration for clopidogrel therapy in the first audit, and this reduced to only 5% in the re-audit. This clearly demonstrates a failure to implement the consensus guidelines within that individual trust.
Next steps for the cardiac prescribing forum will be to look at prescribing of aspirin and clopidogrel dual therapy in primary care. The aim will be to ensure that, where acute care has communicated a duration for therapy at discharge, this is adhered to in primary care.
In addition, the forum will aim to provide additional support to the acute trust where clopidogrel durations are not yet consistently communicated on the discharge summary. A re-audit will be planned to ensure the progress demonstrated across the sector is sustained.
Conflict of interest
The SELCSN have developed an Industry Working Group, of which sanofi-aventis is a member. HW has received honoraria and attended advisory board meetings for sanofi-aventis
Key messages
- A sector-wide approach to clopidogrel prescribing across South East London has improved consistency in prescribing through:
- Engagement of a multidisciplinary team to identify key issues with clopidogrel prescribing
- Development and implementation of consensus prescribing guidelines for the use of clopidogrel in cardiac patients across primary and secondary care
- Encouraging acute trusts to ensure primary care are informed of the indication for and duration of clopidogrel and aspirin dual therapy following discharge
References
- National Institute for Health and Clinical Excellence. Clopidogrel in the treatment of non-ST segment elevation acute coronary syndromes. NICE Technology Appraisal 80. London: NICE, 2004. Available from: http://www.nice.org.uk/nicemedia/pdf/TA080fullguidance.pdf
- National Institute for Health and Clinical Excellence. NICE Technology Appraisal 80: Clarification of Recommendation 1.3. London: NICE, October 2004.
- National Institute for Health and Clinical Excellence. Secondary prevention in primary and secondary care for patients following a myocardial infarction. NICE Clinical Guideline 48. London: NICE, 2007.
- Thomas M on behalf of British Cardiovascular Interventional Society council. BCIS council statement on stent thrombosis and drug eluting stents. BCIS, Autumn 2006. Available from: www.bcis.org.uk [accessed 11 December 2007]
