Contrast enhancement cardiac magnetic resonance (CE-CMR) is a new tool for the assessment of myocardial viability. The technique uses an inversion-recovery prepared T1-weighted gradient-echo pulse sequence after the intravenous administration of a gadolinium-chelate (Gd). Gd diffuses into the interstitium but not the myocardial cells, hence, infarcted myocardium has an increased concentration of Gd resulting in hyper-enhancement. CE-CMR was validated in animal models,1 and human studies confirmed that the technique identifies the presence and extent of myocardial infarction in addition to predicting reversible myocardial dysfunction in patients undergoing revascularisation.2-4 The CE-CMR images consist of short-axis and long-axis slices of the left ventricle (LV) and are analysed based on the 17-segment model of the LV. The non-viable myocardial tissue is hyper-enhanced and white. The degree of the transmurality of the contrast enhancement (CE) in each segment is scored, based on outcome data, where <25% transmurality is highly likely to recover function, 25–50% transmurality is potentially viable with 50% chance of recovering function, and >50% transmurality is unlikely to be viable.2-4 We present two cases, which illustrate the usefulness of this technique in detecting viable myocardium and facilitating the clinical decision-making process for revascularisation.
Case 1
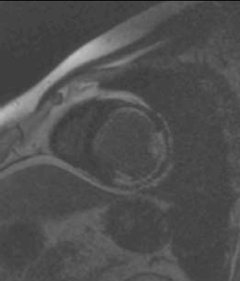
Mr K P is a 45-year-old man who presented to our hospital with symptoms of exertional breathlessness. His LV function was found to be severely impaired on echocardiography and it was initially thought that he had ‘dilated cardiomyopathy’. As he had a strong family history of coronary artery disease and was an ex-smoker, he underwent X-ray coronary angiography. He was found to have an occluded left anterior descending (LAD) artery, severe stenosis of the proximal circumflex and mild disease of the right coronary artery (RCA), which provided collateral blood supply to the LAD. At first, percutaneous coronary intervention (PCI) to his circumflex artery was considered, however, a CE-CMR viability study was performed first (figure 1). It demonstrated two non-viable segments in the anterolateral and inferolateral walls (circumflex territory). The anterior wall was viable with only a subendocardial infarction, with 25% transmurality of CE. He underwent coronary artery bypass grafting (CABG) and made an uneventful recovery.
Case 2
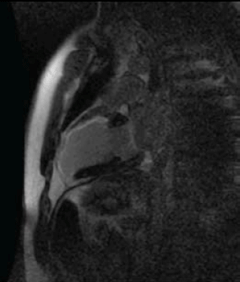
Mr G F is a 38-year-old man who was referred from a district general hospital for PCI to his LAD artery. He presented with a narrow complex tachycardia, which was treated with intravenous adenosine. His electrocardiogram (ECG) (post cardioversion) showed anterior leads T-wave inversions and his troponin was 2.14µg/L. He underwent diagnostic X-ray coronary angiography, which was reported to show a hypokinetic anterior wall and apex, the LAD was irregular and contained thrombus within it, the circumflex and the RCA were normal. On arrival in the cardiac centre, he was asymptomatic and on reviewing his coronary angiogram the LV anterior wall and apex were judged to be akinetic. A CE-CMR viability scan was performed (figure 2). This showed no viable myocardial segments in the LAD territory and, hence, he was managed medically.
Discussion
The above cases demonstrate how the diagnostic information provided by CE-CMR can enhance and improve the decision-making process for revascularisation. This is particularly relevant in patients who do not have anginal symptoms, have significantly impaired LV function, and whom are at a relatively higher surgical risk. The limited data that exist suggest that heart failure patients with viable myocardium are more likely to benefit from revascularisation, in terms of improvement in symptoms and survival.2 Randomised controlled trial data to prove such benefit are expected in 2011 from the CABG versus medical therapy arm of the Surgical Treatment for Ischaemic Heart Failure (STICH) trial. Equally important is to confirm that a territory of the LV is completely infarcted, as there would be no benefit from revascularisation. The Occluded Artery Trial showed a trend towards harm in late revascularisation following myocardial infarction.5 CE-CMR can be utilised to clarify whether there is viable myocardium before considering revascularisation
Conflict of interest
None declared.
References
- Kim RJ, Fieno DS, Parrish TB et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002.
- Kim RJ, Wu E, Rafael A et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53.
- Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation 2001;104:1101–07.
- Mahrholdt H, Wagner A, Holly TA et al. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 2002;106:2322–7.
- Hochman JS, Lamas GA, Buller CE et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006;355:2395–407.
