There are no guidelines on the practice of antibiotic prophylaxis in pacemaker implantation resulting in wide variation in practice. We sought to investigate this and identify areas for further study and improvement. Using an email questionnaire, followed up with a telephone call if no response, all 121 adult National Health Service hospitals in England implanting pacemakers were asked about use of systemic prophylactic antibiotics at implantation. Data were obtained from 61 hospitals (50.4% of total contacted), covering a wide geographic distribution.
Fifty-six of the 61 units (92%) used prophylactic antibiotics at the time of implantation. Fifty-five of these (98%) covered Staphylococcus aureus. Thirty-one (51%) used intra-pocket gentamicin at implantation. Forty-three of the 61 units (71%) used systemic prophylactic antibiotics post-implantation. All 43 covered Staph. aureus. Duration of use ranged from six hours to seven days.
Systemic prophylactic antibiotics at the time of pacemaker implantation are effective and recommended. Further study is required into the effectiveness of intra-pocket antibiotics despite 51% of centres using them. Post-implant antibiotic regimens showed wide variation, requiring further study. Excessively broad-spectrum antibiotics or those implicated in antibiotic-associated infections, should be avoided where possible. Our study is the first observational study of its kind.
Introduction
Approximately 25,000 pacemakers are implanted per year in the UK, with the hardware cost alone exceeding £43 million.1 With an increasingly ageing population, implantation rates are rising. Pacemaker pocket infection is a feared and potentially life-threatening complication after implantation, and can require device extraction. It occurs in up to 5% of implants, and the severe form, pacemaker-related endocarditis was shown to have an overall mortality of 27% after a mean follow-up of 20 months.2,3 Staphylococcus species (especially Staph. aureus, and to a much lesser extent coagulase-negative Staphylococci, such as Staph. epidermis) are the culprits in the vast majority of cases where organisms have been isolated, with rates varying between 56–100% in studies.4-12
Systemic antibiotic prophylaxis is thus routinely undertaken at the time of pacemaker implantation. We undertook a systematic review of all randomised trials to date studying the effectiveness of this practice. Detailed Medline and Pubmed searches were undertaken, which identified a total of nine studies, undertaken between 1981 and 2009.2,4,5,12-20
Da Costa and colleagues undertook a meta-analysis of all seven randomised-controlled trials until 1998, studying the effectiveness of systemic prophylactic antibiotics in reducing pacemaker-related infections. This showed their use at the time of implantation significantly reduced the incidence of implant-associated infections (combined odds ratio 0.256, p=0.046).2,4,13-20 In 2000, Klug and colleagues demonstrated a significant reduction in infections related to implantation of pacemakers (n=5,866) and cardioverter-defibrillators (n=453) in a study of 6,319 insertions with systemic prophylactic antibiotics at implantation (odds ratio 0.4).5
Most recently, Oliveira and colleagues published data from the first randomised, double-blind, placebo-controlled trial establishing the benefit of systemic antibiotics at the time of implantation in preventing device-related infection. In a study of 649 consecutive patients randomised to either 1 g intravenous cefazolin or placebo (saline) with follow-up at day 10, and months one, three and six, prophylactic antibiotic use was shown to significantly reduce the incidence of infection (relative risk 0.19, p=0.016).12
However, there are no guidelines on systemic prophylactic antibiotic use in pacemaker implantation, and the decision on whether to use them is usually at the discretion of the operator. In addition, we found no trials comparing systemic prophylactic antibiotic regimens, no studies of intra-pocket antibiotic use, and only one study of prophylactic antibiotic use post-implantation.6 In light of this, we elected to undertake a survey of prophylactic antibiotic use in permanent pacemaker implantation in adult cardiology units in England.
The aim of this observational research was to ascertain whether centres were using systemic prophylactic antibiotics at the time of implantation, as shown to be effective in studies; and, if so, whether they would cover the key organism, Staph. aureus. The use of intra-pocket antibiotics and practice of prophylactic antibiotic use post-implantation was evaluated, in order to identify areas for further study and improvement.
Materials and methods
Inclusion criteria
All 121 adult National Health Service (NHS) cardiology hospital units undertaking pacemaker implantation were identified in England. Private hospitals and paediatric cardiology units were excluded.
Data collection
An email questionnaire investigating antibiotic prophylaxis at the time of implantation and post-implantation was sent to the cardiology registrars in the units in 2008. Where there was no reply, we then telephoned the remaining units to obtain the questionnaire data. We initially tried contacting a cardiology registrar. Where this was unsuccessful, we then spoke to the most senior cardiology sister available.
Antibiotic prophylaxis at the time of implantation
We investigated whether systemic prophylactic antibiotics were used at the time of implantation. Where used, the regimen and route of administration were recorded. We then looked at whether the regimen would cover the key organism, Staph. aureus. In units where our surveys revealed that no systemic prophylactic antibiotics were used, we contacted them again by telephone two weeks later to re-confirm this. Finally, we investigated whether intra-pocket gentamicin was administered at implantation.
Antibiotic prophylaxis post-implantation
We investigated whether post-implantation antibiotics were used. Where used, the antibiotic regimen, route of administration and duration of administration were recorded. Again, Staph. aureus cover was investigated.
Statistical analysis
Data analysis was represented as numbers and proportions.
Results
Study cohort
Data were obtained from 61 units (51% of adult NHS cardiology units in England), covering a widespread geographical distribution (figure 1). Initial email replies were received from 20 registrars, covering 15 units. Data from the other 46 units was then obtained via telephone questionnaire. Six replies were from the same unit, providing an insight into practices within a large single unit.
Antibiotic prophylaxis at the time of implantation
Fifty-six of the 61 units (91.8%) used prophylactic antibiotics at the time of implantation. All 56 antibiotic regimens (100%) provided cover against Staph. aureus. Forty-eight (86%) were given intravenously, and eight were given orally. The most common regimen was flucloxacillin alone. Nine different antibiotic regimens were used (table 1).
Thirty-one units (50.8%) used intra-pocket gentamicin at the time of implantation, one (1.6%) used vancomycin, and the remaining 29 (47.6%) used no intra-pocket antibiotics. All five units that did not use systemic prophylactic antibiotics at the time of implantation used intra-pocket gentamicin.
All six replies from the same unit stated that systemic antibiotics (all covering Staph. aureus) and intra-pocket gentamicin were used at the time of implantation, and five out of six indicated uniform post-implantation antibiotic use.
Antibiotic prophylaxis post-implantation
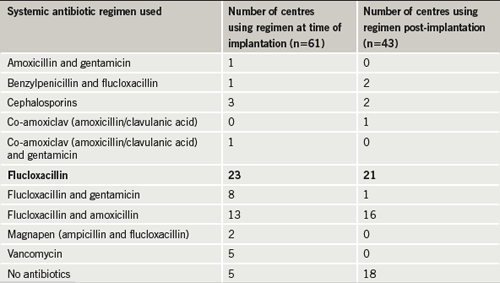
Forty-three of the 61 units (70.5%) used systemic prophylactic antibiotics post-implantation. All 43 provided activity against Staph. aureus. Again, the most common regimen was flucloxacillin alone. Six different antibiotic regimens were used (table 1). Thirty-six (83.7%) were given orally and seven (16.3%) were given intravenously. Where used, the duration of use ranged from six hours to seven days (mean 54.5 hours [2.25 days], median 48 hours), with five days the most common. Five of the six replies from the same unit stated that systemic antibiotics were used post-implantation.
Discussion
Antibiotic prophylaxis at the time of implantation
We have shown that at the time of implantation, a high proportion (56 of 61, 91.8%) of centres are using systemic antibiotic prophylaxis, as shown to be effective and strongly advised in previous studies. However, almost 9% of centres used no systemic antibiotic prophylaxis. These centres did, however, give intra-pocket antibiotics with activity against Staph. aureus. It has been shown that Staph. aureus is the culprit species in the vast majority of pacemaker infections. Encouragingly, all of the above (56) centres used beta-lactam antibiotics with activity against this organism.2,5,12-20
There are no randomised-controlled trials studying the effectiveness of prophylactic intra-pocket gentamicin in pacemaker implantation. However, half of the centres used this approach in our study. Gentamicin covers against Gram-negative organisms and has activity against some Gram-positive organisms including Staph. aureus.13 Indeed, the use of gentamicin-coated implants and gentamicin-containing cements in orthopaedic implants have been shown to reduce the incidence of implant-associated osteomyelitis, especially due to Staph. aureus.21,22 Gentamicin coatings have been used to provide a reduction in infections related to neurosurgical implants (e.g. shunts).23 The effectiveness of intra-pocket gentamicin in preventing pacemaker-pocket infection needs to be further studied.
Antibiotic prophylaxis post-implantation
Only 70% of centres used post-implantation prophylactic systemic antibiotics. All of these provided good cover against Staph. aureus with the use of beta-lactam antibiotics. However, this was much lower than use at the time of implantation (cf. 91.8%). Although six of the seven studies included in the meta-analysis by Da Costa et al. not only gave antibiotics at the time of implantation, but also for varying durations post-implantation, we could find no studies specifically investigating the effectiveness of using prophylactic antibiotics post-implantation.4 There is a real need for such studies.
Antibiotic regimens used and their duration
Nine different systemic antibiotic regimens were used at the time of implantation, and six post-implantation. Unless studies are undertaken comparing specific antibiotic regimens and their efficacy against pacemaker infections, it is difficult to evaluate a superior regimen. It appeared that the use of flucloxacillin alone was the most common regimen. Encouragingly, all of the regimens used provided cover against Staph. aureus. In addition, it is important to appreciate local guidelines and differing regimens between hospitals, and, thus, we feel the key aspect of a successful prophylactic regimen is good activity against Staph. aureus as a minimum.
However, the need for good cover against Staph. aureus must be balanced against the risk of antibiotic-associated infections, especially Clostridium difficile associated diarrhoea (CDAD). National Office of Statistics data illustrate that the mortality rate from CDAD in the UK has increased on a yearly basis, from 11.4 per million population in 1999 to 84.9 per million population in 2007.24 Of the antibiotics used in our survey, and in the nine randomised studies undertaken, cephalosporins have been shown to be among the most closely implicated in the development of CDAD, followed by amoxicillin and ampicillin. Narrow-spectrum penicillins, such as flucloxacillin, and aminoglycosides, such as gentamicin, cause CDAD only rarely.25-27 Even a single dose of cephalosporins, such as cefazolin, as used by Oliveira and colleagues in the most recent study, has been known to cause CDAD.28-29
It is, thus, important that excessively broad-spectrum antibiotics, or those known to be strongly linked with the development of CDAD, should be avoided where possible. It could be argued that since Staph. aureus is the major organism involved in pacemaker-associated infections and flucloxacillin provides excellent cover against this, with a very low incidence of CDAD, that cover with flucloxacillin alone is sufficient. However, we accept that five of the 13 infections in the recent study by Oliveira and colleagues were due to coagulase-negative Staphylococci, which are often flucloxacillin resistant. Gentamicin has good activity against these organisms. Therefore, it is possible that the addition of intra-pocket gentamicin, as already used by some operators, to systemic administration of flucloxacillin could provide effective prophylaxis against Staph. aureus with a potentially significantly lower risk of CDAD.12,30
In particular to post-implantation antibiotic use, there was wide variation in the duration of treatment in our study, ranging from six hours to seven days (168 hours). We could find only one study investigating duration of post-implantation antibiotics, and this looked at short-term (two days) versus longer-term (seven days) use. It described that a short course is just as effective as a longer course in preventing pacemaker infections.6 There needs to be further study looking specifically at durations of post-implantation antibiotic use. This would potentially not only improve prevention of pacemaker infections, but also prevent excessively long use of post-implantation antibiotics. This is, again, especially important with the increasing prevalence of antibiotic-associated infections seen today.
Regarding the route of administration, 86% of units using systemic prophylactic antibiotics at the time of implantation used intravenous antibiotics. Post-implantation, 16% of units using such antibiotics administered them intravenously. Intravenous antibiotics may well provide faster absorption and protection, however, this needs to be balanced with the risk of cannula-associated infections, which could potentially lead to pacemaker infection. It may be argued that since a peripheral cannula is inserted routinely prior to pacemaker implantation, then intravenous administration provides no additional infection risk. There is a need for studies of efficacy and iatrogenic infections related to the routes of administration of prophylactic antibiotics in pacemaker implantation.
Study limitations
This study is limited by the fact that we were unable to obtain data from almost half of the target centres for data collection.
Conclusion
Systemic prophylactic antibiotics at the time of pacemaker implantation have been shown to be effective in studies and strongly recommended. Our study suggests that adult pacemaker centres in England are adhering to this well, with over 91% of units using systemic prophylactic antibiotics at implantation.2,4,5,13‑20 Encouragingly, all centres used antibiotics providing cover against Staph. aureus, the main culprit organism.
There is a lack of research into a number of aspects regarding antibiotic prophylaxis in pacemaker implantation. These include studies comparing systemic antibiotic regimens at the time of prophylaxis. Pacemaker infection rates in centres not using systemic antibiotic prophylaxis at implantation need to be compared with those using standard practice. Further study is required as to the effectiveness of intra-pocket antibiotic use, despite 51% of centres using this approach. Post-implant antibiotic regimens showed wide variation in duration, again, requiring further study for optimal strategy.
Acknowledgement
We are grateful to the hospitals that responded to our questionnaire and telephone calls for data collection.
Conflict of interest
None declared.
Key messages
- Systemic prophylactic antibiotics at the time of pacemaker implantation are strongly recommended, and centres in England are adhering well to this
- Guidelines on antibiotic prophylaxis in pacemaker insertion, however, are needed
- Regimens should provide good activity against Staph. aureus while avoiding broad-spectrum antibiotics or those associated with antibiotic-associated infections
- Studies into the efficacy of intra-pocket and post-implantation antibiotics are needed
References
1. National Institute for Clinical Excellence. Technology Appraisal 088. Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block. London: NICE, 2005.
2. Bluhm G, Jacobson B, Julander I et al. Antibiotic prophylaxis in permanent pacemaker surgery: a prospective study. Scand J Thor Cardiovasc Surg 1984;18:227–34.
3. Klug D, Lacroix D, Savoye C et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation 1997;95:2098–107.
4. Da Costa A, Kirkorian G, Cucherat M et al. Antibiotic prophylaxis for permanent pacemaker insertion: a meta-analysis. Circulation 1998;97:1796–801.
5. Klug D, Balde M, Pavin D et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators. Circulation 2007;116:1349–55.
6. Dwivedi S, Saran R, Khera P et al. Short-term (48 hours) versus long-term (7 days) antibiotic prophylaxis for permanent pacemaker implantation. Indian Heart J 2001;53:740–2.
7. Sohail M, Uslan D, Khan A et al. Risk factor analysis of permanent pacemaker insertion. Clin Infect Dis 2007;45:166–73.
8. Villamil I, Rodriguez M, Van den Eynde A et al. Permanent transvenous pacemaker infections: an analysis of 59 cases. Eur J Intern Med 2007;18:484–8.
9. Catanchin A, Murdock C, Athan E. Pacemaker infections: a 10-year experience. Heart Lung Circ 2007;16:434–9.
10. Da Costa A, Kirkorian G, Isaaz K et al. Secondary infections after pacemaker implantation. Rev Med Interne 2000;21:256–65.
11. Mounsey J, Griffith M, Tynan M et al. Antibiotic prophylaxis in permanent pacemaker implantation: a prospective randomized trial. Br Heart J 1994;72:339–43.
12. de Oliveira JC, Martinelli M, D’Orio Nishioka A et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2009;2:29–34.
13. Chambers S. Diagnosis and management of staphylococcal infections of pacemakers and cardiac defibrillators. Intern Med J 2005;35(suppl 2):S63–S71.
14. Jacobson B, Bluhm G, Julander I, Nord CE. Coagulase-negative staphylococci and cloxacillin prophylaxis in pacemaker surgery. Acta Pathol Microbiol Scand 1983;91:97–9.
15. Mounsey JP, Griffith MJ, Gold RG, Bexton RS. Antibiotic prophylaxis reduces reoperation rate for infective complications following permanent cardiac pacemaker implantation: a prospective randomized trial. Circulation 1993;88(suppl I):I-19. Abstract.
16. Lüninghake F, Gottschalk A, Stierle U, Potratz J, Sack K, Diederich KW. Antibiotic prophylaxis for pacemaker implantation: a prospective randomized trial in 302 patients. PACE 1993;16:1138. Abstract.
17. Bluhm G, Norlander R, Ransjö U. Antibiotic prophylaxis in pacemaker surgery: a prospective double blind trial with systemic administration of antibiotic versus placebo at implantation of cardiac pacemakers. PACE 1986;9:720–6.
18. Ramsdale DR, Charles RG, Rowlands DB, Singh SS, Gautam PC, Faragher EB. Prophylactic antibiotics for cardiac pacemaker implantation: a prospective randomized trial. PACE 1984;7:844–9.
19. Glieca F, Luciani N, Di Giammarco G et al. Role of antibiotic prophylaxis in pacemaker implantation. Minerva Cardioangiol 1987;35:549–52.
20. Muers MF, Arnold AG, Sleight P. Prophylactic antibiotics for cardiac pacemaker implantation: a prospective trial. Br Heart J 1981;46:539–44.
21. Josefsson G, Lindberg L, Wiklander B. Systemic antibiotics and gentamicin-containing bone cement in the prophylaxis of postoperative infections in total hip arthroplasty. Clin Orthop Relat Res 1981;159:194–200.
22. Price JS, Tencer AF, Arm DM, Bohach GA. Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res 1996;30:281–6.
23. Rushton DN, Brindley GS, Polkey CE, Browning GV. Implant infections and antibiotic-impregnated silicone rubber coating. J Neurol 1989;52:223–9.
24. National Office of Statistics. Clostridium difficile. Available from: http://www.statistics.gov.uk/cci/nugget.asp?id=1735 [accessed August 2008].
25. Aronsson B, Möllby R, Nord CE. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980–1982. J Infect Dis 1985;151:476–81.
26. Ho M, Yang D, Wyle FA, Mulligan ME. Increased incidence of Clostridium difficile-associated diarrhea following decreased restriction of antibiotic use. Clin Infect Dis 1996;23(suppl 1):S102–S106.
27. Starr JM, Martin H, McCoubrey J et al. Risk factors for Clostridium difficile colonisation and toxin production. Age Ageing 2003;32:657–60.
28. Ambrose NS, Johnson M, Burdon DW et al. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J Antimicrob Chemother 1985;15:319–26.
29. Privitera G, Scarpellini P, Ortisi G et al. Prospective study of Clostridium difficile intestinal colonization and disease following single-dose antibiotic prophylaxis in surgery. Antimicrob Agents Chemother 1991;35:208–10.
30. Mohan U, Jindal N, Aggarwal P. Species distribution and antibiotic sensitivity pattern of coagulase negative staphylococci isolated from various clinical specimens. Indian J Med Microbiol 2002;20:45–6.
