A new and exciting ‘journey’ in interventional cardiology began more than 20 years ago with the introduction of intracoronary stenting.
Introduction
Developments along the way have included better patient selection, improved peri-procedural management of patients and, with newer-generation drugs and devices, better results. Recent hurdles have been confronted, including left main stem disease, complex bifurcation lesions and total chronic occlusions. Similarly, primary percutaneous coronary intervention (PCI) has become the treatment of choice in acute myocardial infarction. Challenges remain, however, including restenosis. The fine balance between thrombosis and haemostasis demands that we provide more effective and predictable antiplatelet strategies to optimise risk reduction.
Newer approaches are being developed, including stents made of biological materials and biodegradable stents, along with refinements and adjuncts in pharmacology, to provide more potent and selective inhibition of the many pathways of platelet activation and aggregation. We may also be entering an era of routine genotyping and point-of-care platelet function testing. Exciting times! The purpose of this supplement is to review the “state of the art” and see where the journey has brought us, and also to look a little at what may lie ahead. In this first section we will review newer terms and definitions, and we will move on to look in detail at some of the remaining clinical challenges such as coronary artery disease in patients with diabetes.
New terms and approaches to risk management
Acute coronary syndrome (ACS) is now the label which describes a range of symptoms extending from unstable angina to myocardial infarction. As more sensitive and specific serological biomarkers and more precise imaging techniques have developed, ever smaller amounts of myocardial necrosis are now detectable. Therefore previous definitions of myocardial infarction have been re-evaluated, a process which has implications for clinicians for risk-stratifying high- versus low-risk groups; for health care delivery systems for future planning and resource utilisation; and also for design and analysis of clinical trials.
A universal definition of myocardial infarction was developed by a joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation (ESC/ACCF/AHA/WHF) task force.1 Now, the term myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting consistent with myocardial ischaemia. Criteria include detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit together with evidence of myocardial ischaemia with at least one of the following:
- Symptoms of ischaemia
- ECG changes indicative of new ischaemia (new ST-T changes or new left bundle branch block)
- Development of pathological Q waves in
the ECG - Imaging evidence of new loss of viable myocardium or new regional wall
motion abnormality.
The preferred biomarkers are troponin T or I, which have nearly absolute myocardial tissue specificity as well as high clinical sensitivity, reflecting even microscopic zones of myocardial necrosis.1
Acute ST-segment elevation myocardial infarction (STEMI) is associated with coronary artery occlusion and progressive necrosis of the myocardial tissue. In non-ST segment elevation myocardial infarction (NSTEMI), thrombus does not occlude the lumen completely, or perhaps blocks it intermittently.2 Some myocardial necrosis occurs in these circumstances also. But when myocardial ischaemia is present without myocardial necrosis (normal serum troponin level), the clinical syndrome is termed unstable angina. The ESC definition of ACS is illustrated in figure 1.3
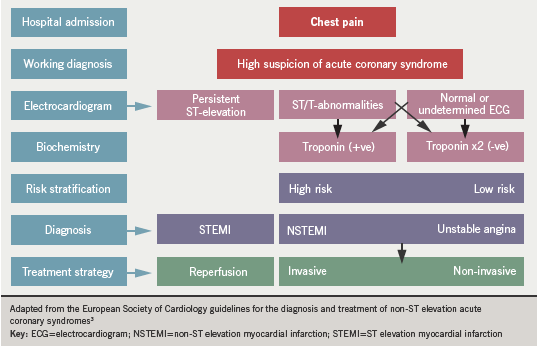
Although mortality from myocardial infarction has declined in recent years in the UK, the number of people admitted to hospital with unstable angina and NSTEMI (NSTE-ACS) has shown less of a decline. Although STEMI historically was considered to produce worse outcomes, we now know that six-month mortality from NSTE-ACS is comparable to that of STEMI patients.3 Indeed, data suggest that six-month mortality may be worse in NSTEMI.4 The management of these patients, therefore, remains a high priority, particularly in face of worrying trends in the incidence of obesity and diabetes. Patients with ACS also have widely varying risks of mortality and other cardiovascular events, both in-hospital and after discharge.2 Many factors have been shown to be predictive of adverse outcome during ACS, including advancing age, raised heart rate, the magnitude in rise of biomarkers of myocardial injury, and diabetes mellitus.2
A single risk factor may not provide a reliable assessment of outcome. In clinical practice there has been a tendency to use serum troponin levels for patient risk stratification but “serum troponin does not accurately measure risk in individual patients, particularly when used as a dichotomous outcome”.2 A number of risk scoring systems have been developed to predict outcome in ACS patients. Many are derived from clinical trial populations (such as PURSUIT5 and PREDICT6), which have generally excluded patients at highest risk; others (such as GRACE7) have been derived from large patient databases in an attempt to obtain a population with a broader spectrum of risk. None of these risk models is clearly superior, according to the National Institute for Health and Clinical Excellence (NICE) 2010 guideline. 2 The same document continues: “Clinical assessment alone may not accurately reflect the patient’s risk. There is potential for a systematic approach to risk assessment (that would) result in more accurate estimation of risk and more appropriate intervention”2.

References
1. Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J 2007; 28:2525–38.
2. The National Institute for Health and Clinical Excellence (NICE). Unstable angina and NSTEMI: the early management of unstable angina and non-ST-segment-elevation myocardial infarction. Clinical guideline CG94. London: NICE, 2010.
3. Guidelines for the diagnosis and treatment of non-ST elevation acute coronary syndromes. Task Force for diagnosis and treatment of non-ST elevation acute coronary syndromes of the European Society of Cardiology. Bassand JP, Hamm CW, Ardissino D et al. Eur Heart J 2007;28:1598–660.
4. Nair SB, Chacko SM, Dixit A et al. Comparisons of patients’ demographics, in-hospital and three-year mortality rates and independent predictors of death in ST-elevation versus non-ST-elevation myocardial infarction. An interventional centre experience. Heart 2010;96(suppl 1):A71–A72.
5. Boersma E, Pieper KS, Steyerberg EW et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000;101:2557–67.
6. Jacobs DR, Kroenke C, Crow R et al. PREDICT: A simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation 1999;100:599–607.
7. Fox KA, Dabbous OH, Goldberg RJ et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndromes: prospective multinational observational study (GRACE). BMJ 2006;333:1091.
