Treatment options in ACS include optimised medical therapy, intervention and surgical revascularisation.
Introduction
While primary PCI, rather than thrombolysis, is now the reperfusion treatment of choice for STEMI, the majority of patients coming for revascularisation in the UK have stable coronary disease or NSTE-ACS. In the treatment of NSTE-ACS, first principles involve the selection of patients for diagnostic angiography followed by either PCI or coronary artery bypass grafting (CABG). Rates of PCI are increasing annually in the UK, which, in part, is a reflection of greater awareness of coronary artery disease, its earlier diagnosis and treatment in the ageing population.
This section looks at coronary intervention in general, how PCI activity in the UK compares with other European countries, and trends in the mode of vascular access. We also look at outcomes following PCI compared with CABG and latest UK guidance in this area.
Percutaneous coronary intervention statistics in the UK
Audit returns data from the British Cardiovascular Intervention Society (BCIS)1 show the current state of PCI in the UK. It is becoming an increasingly common procedure: latest data from 2008 show that 105 centres in the UK performed percutaneous coronary intervention (PCI).1 PCIs were performed in five NHS and one private facility in Scotland; in 76 NHS and 16 private facilities in England; in three hospitals in Northern Ireland; and in two in Wales.1 The total number of PCIs for the UK for 2008 was 80,331: the vast majority (78,077) in NHS hospitals and 2,254 in the private sector.1 Figure 1 expresses BCIS data showing that both the number of PCIs performed and the number of PCIs performed per million population are gradually increasing each year. In addition, 54 of the 87 NHS units now perform day-case PCI.1
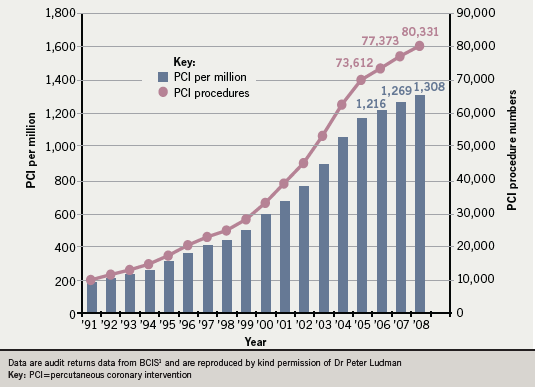
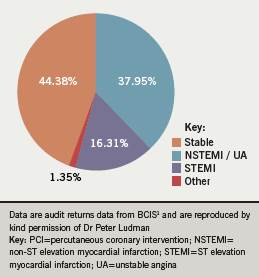
Figure 2 shows the indications for PCI during 2008.1 The “other” indications include bailout and hybrid procedures.
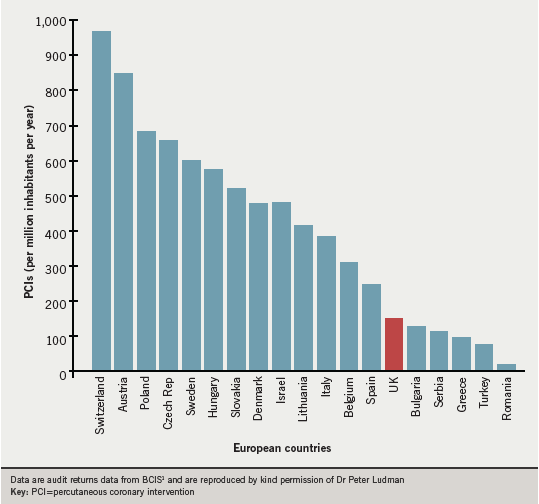
In all, 11,784 PCIs were performed for STEMI, most of them (n=9,224) primary PCI and the others rescue PCI.1 This gives an overall primary PCI rate of 150 per million population in the UK, a lower figure than those from many European countries (figure 3).1
Compared to 2007, interventionists made increasing use of thrombectomy, laser, rotablation, intravascular ultrasound and pressure wires but decreasing use of cutting balloons and distal protection.1
Femoral and radial access routes for PCI: take-up and results
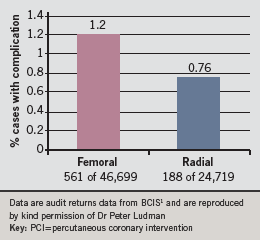
Historically PCI has been carried out using femoral artery access. Over the years, the mode of vascular access in PCI has changed to reduce procedural complications. BCIS data for 2008 show how radial artery access for PCI is slowly becoming more widespread in the UK.1 It was used for 28.1% of cases in 2007 and 34.61% of cases in 2008, in roughly one third of patients presenting with stable coronary syndromes, acute coronary syndromes (not STEMI) and those who underwent primary PCI.1 Fewer complications were reported after use of the radial route compared to the femoral route, see figure 4.1 BCIS also reports that there appears to be no significant difference in door-to-balloon (DTB) time between the two routes. The mean DTB time for the 2,511 procedures using femoral artery access was 66.02 minutes, compared to 63.16 minutes for the 976 procedures using the radial artery (p=0.08).
The patient pathway
Symptomatic patients who receive PCI may already be in hospital. More often, they will be admitted from the community, either directly to a PCI centre or having first been admitted to a non-PCI centre and then transferred. Data indicate that the time to PCI is shorter when the patient is admitted directly to a PCI centre. Thus, for NSTEMI/unstable angina and convalescent STEMI, the median door to PCI time is 2.96 days. In contrast, it is 4.2 days in total for patients who follow the latter route: this is composed of 3.2 days for door 1 (non-PCI centre) to door 2 (PCI centre) and 1.0 days for door 2 to PCI.1 (These figures may be compared with NICE clinical guideline 94, which recommends that coronary angiography [with follow-up PCI if indicated] should be offered within 96 hours of first admission to hospital to patients at intermediate or higher risk of adverse cardiovascular events.)2
Outcomes of PCI
For 2008, BCIS reports there was a 91.8% procedure success rate for all PCIs.1 Adverse outcomes include death (overall mean in-hospital mortality rate 1.03%), cerebrovascular accident (0.08%) and need for emergency CABG (0.07%).1
Table 1 shows an analysis of outcomes for 2008 for PCIs performed for NSTEMI, STEMI, primary PCI and rescue PCI.1 It can be seen that success rates are very high—over 93% for NSTEMI, unstable angina and STEMI in the absence of shock, and over 91% for primary and rescue PCI. The mortality may be risk-stratified by syndrome. The overall in-hospital mortality rate is 1.03% but the mortality is far lower among those with an elective PCI (0.13%), low in those treated by PCI for unstable angina or NSTEMI (0.62%), and higher in those treated with primary or rescue PCI (4.1% and 4.7%, respectively).1
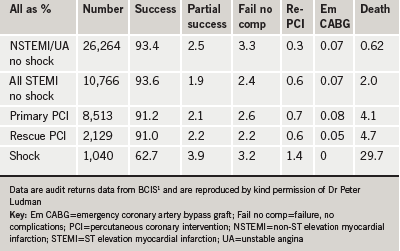
There are also gender differences in in-hospital mortality: overall mortality is higher among women (1.36% versus 0.95%). The percentage mortality is similar between men and women for those with elective procedures but again is higher in women for those with STEMI (5.44% versus 3.58%).1 This highlights that the difference in outcome between genders is greater in the higher-risk procedures.
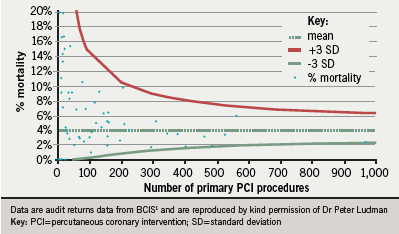
The mortality for primary PCI varies with the number of interventions performed. Figure 5 illustrates the mortality versus volume funnel, showing that while there were no worrying ‘outliers’, and that there is wide variation in reported mortality between centres, there appears to be a trend of lower complications at higher volumes.1 This has led to calls for PCI to be carried out in higher volume specialist centres.
Primary PCI as routine treatment for STEMI
With the introduction of specialist centres has come national policy to promote primary PCI rather than thrombolysis as a treatment of choice for STEMI.3 2008 BCIS data show there were either daytime-only or round-the-clock primary PCI in 50 NHS centres and round-the-clock in 28 NHS centres.1 For patients admitted directly to PCI units, the mean door-to-balloon (DTB) time was 53.8 minutes, with 81.3% of all patients having a DTB time below 90 minutes. For this same group of patients, the mean call-to-balloon time (CTB) was 116.6 minutes, with 78.8% of all patients having a CTB time below 150 minutes.1
These figures may be compared with international figures.4 Data from 5,170 patients with STEMI enrolled in GRACE from 2003 to 2007 were examined. The median elapsed time from first hospital presentation to primary PCI was 86 minutes (interquartile range 53-135 minutes), with no significant changes in delay times to treatment over the years under study. The strongest predictors of prolonged delay time were geographic location and patient transfer.
Crossroads decision: PCI versus CABG in NSTE-ACS
For non-STEMI patients, cardiologists now have to decide on optimum treatment and which pathway to follow.
Guidance for NSTE-ACS was published in March 2010 in the UK by the National Institute for Health and Clinical Excellence (NICE).2 The guideline was developed by the National Clinical Guideline Centre of the Royal College of Physicians on behalf of NICE. Part of this guidance considered PCI versus CABG and concluded that current evidence supports the use of both revascularisation strategies – the selection of which procedure to use in an individual patient will depend on factors such as the extent and severity of coronary disease (which presently would require diagnostic coronary angiography), left ventricular function, estimated risk, co-morbidity and patient choice. Multi-disciplinary meetings may be used to help determine this. Comparative cost-effectiveness of the two procedures is uncertain given the lack of outcome data: it will need to take into consideration any survival advantage and the need for repeat procedures (including drug intervention to prevent re-stenosis).
This guidance was based on the results from four randomised trials in which patients with unstable angina or NSTEMI were randomised to PCI or CABG: ERACI-II,5 AWESOME,6 SoS7 and ARTS8.
In ERACI-II, 450 patients with NSTE-ACS were randomised: at 30 days, CABG patients had higher rates of death, acute myocardial infarction and major adverse cardiac and cerebrovascular events.5 The difference in mortality rates persisted to 33 months but was non-significant at five years. Those who underwent PCI initially had a higher rate of further revascularisation procedures during follow-up. In the AWESOME trial,6 there was no difference in survival between the PCI and CABG groups but more from the PCI group required further revascularisation procedures. A subgroup analysis of SoS,7 and similarly of ARTS,8 showed a non-significant difference in death rates (lower with CABG) after one year, and those in the PCI group had a higher need for repeat revascularisation. All these trials involved only placement of bare-metal stents, which are known to have a higher risk of restenosis than drug-eluting stents. The most significant difference observed in these trials between the two treatment approaches – the difference in need for repeat revascularisation – may therefore over-estimate the advantage of CABG given the increased use of drug-eluting stents.
Caveats to the guidance included the fact that many of the trial data are derived from patients with stable angina; the elderly and those at highest risk may be excluded from trials; and, as surgical techniques and adjunctive therapy have advanced, so the populations of patients considered suitable for either PCI or CABG have changed.
There are still treatment dilemmas. CABG is the obvious choice for a patient with diffuse triple vessel disease or left main stem disease, for example, as shown in the SYNTAX trial.9 In this trial, 1,800 patients with three-vessel or left main coronary disease were randomly assigned to undergo CABG or PCI. Rates of death and myocardial infarction at one year were similar between the two treatment groups; the rate of stroke was increased in the CABG group and the rate of repeat revascularisation was increased in the PCI group. Patients with low or intermediate SYNTAX scores had similar rates of major adverse cardiac or cerebrovascular events; among patients with high SYNTAX scores, the event rate was significantly increased in the PCI group. It is challenging to work out the patient group equally suitable for treatment with either PCI or CABG. NICE recommends that research be conducted into the efficacy and cost-effectiveness of CABG versus PCI in the management of patients with NSTE-ACS.
Early invasive versus conservative management strategies
In order to be considered for PCI or CABG, patients with NSTE-ACS need to be referred for diagnostic angiography. Patients who do not stabilise on optimal medical management, including beta blockers, low molecular weight heparin, plus antiplatelet therapy (conventionally, aspirin and clopidogrel) clearly warrant referral. Many patients, however, settle on medical therapy and historically have been successfully discharged home without any PCI – the “conservative” approach to treatment. Yet, there is evidence that even those who do initially become symptom-free on medical therapy might benefit from revascularisation – the “invasive” approach.
The NICE 2010 guideline considered five trials from the coronary stent era that compared early invasive with conservative strategies for management of NSTE-ACS (FRISC II, TACTICS-TIMI 18, VINO, RITA-3 and ICTUS).10-14 An invasive strategy was found to significantly decrease the composite of death and myocardial infarction at 6-12 months’ follow-up and to reduce the long-term rate of re-hospitalisation. Procedure-related myocardial infarction was significantly increased in the invasive arm. There was no difference in mortality according to whether angiography was performed less than 24 hours or more than 48 hours from randomisation.
Data from FRISC II10 and RITA-313 suggest that the benefit of an invasive strategy is mostly in higher-risk people. ICTUS14 suggested that an early invasive strategy did not confer benefit but in this trial the conservative arm had a high rate of early angiography and revascularisation. Appropriate use of GP IIb/IIIa inhibitors reduced in-hospital mortality when added to an invasive strategy (TACTICS-TIMI 18 and ICTUS trial data).11,14 People randomised to an early invasive strategy had better quality of life scores at six and 12 month follow-up (FRISC II and RITA-3).10,13
NICE recommendations made are: 2
- patients with a predicted six-month mortality of 3% or less should be managed conservatively, without early coronary angiography, but ischaemia testing should be considered before discharge
- patients who are unstable and those at high ischaemic risk should have an angiogram as
soon as possible after admission - those who have an intermediate or higher
risk of cardiac events (predicted six-month mortality > 3.0%) should be offered angiography/PCI within 96 hours in the
absence of contra-indications
References
1. BCIS audit returns for the year 2008, presented by Peter F Ludman, BCIS National Audit Lead. Available online as: BCIS%20Audit%202008%20for%20web%2016-10-09%20version%201.pdf
2. NICE. Unstable angina and NSTEMI: the early management of unstable angina and NSTEMI. Clinical guideline CG94. London: NICE, 2010.
3. McLenachan JM, Marley C, Machin S. Heart improvement. A guide to improving primary angioplasty. NHS improvement 2009. www.improvement.nhs/uk/heart/reperfusion
4. Spencer FA, Montalescot G, Fox KAA et al; GRACE Investigators. Delay to reperfusion in patients with acute myocardial infarction presenting to acute care hospitals: an international perspective. Eur Heart J 2010;31:1328–36.
5. Rodriguez AE. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary artery bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005;46:582–8.
6. Morrison DA, Sethi G, Sacks J et al. Percutaneous coronary intervention versus coronary artery bypass graft surgery for patients with medically refractory myocardial ischemia and risk factors for adverse outcomes with bypass: a multicenter randomized trial. J Am Coll Cardiol 2001;38:143–9.
7. Zhang Z, Spertus JA, Mahoney EM et al. The impact of acute coronary syndrome on clinical, economic and cardiac-specific health status after coronary artery bypass surgery versus stent-assisted percutaneous coronary intervention: 1-year results from the Stent or Surgery (SoS) trial. Am Heart J 2005;150:175–81.
8. De Feyter PJ, Serruys PW, Unger F et al. Bypass surgery versus stenting for the treatment of multivessel disease in patients with unstable angina compared with stable angina. Circulation 2002;105:2367–72.
9. Serruys PW, Morice M-C, Kappetein P et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72.
10. Anon. Invasive compared with non-invasive treatment in unstable coronary artery disease: FRISC II prospective randomized multicentre study. Lancet 1999;354:708–15.
11. Cannon CP, Weintraub WS, Demopoulos LA et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the GP IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879–97.
12. Spacek R, Widimsky P, Straka Z et al. Value of first-day angiography/angioplasty in evolving non-ST segment elevation MI; an open multicentre randomized trial. The VINO study. Eur Heart J 2002;23:130–8.
13. Fox KA, Poole-Wilson PA, Henderson RA et al. Interventional versus conservative treatment for patients with unstable angina or NSTEMI; the British Heart Foundation RITA 3 randomised trial. Lancet 2002;360:743–51.
14. De Winter R, Windhausen F, Cornel JH et al. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med 2005;353:1095–104.
