Midodrine is a sympathomimetic agent used in the treatment of hypotension resulting from various aetiologies. Debate around the use of midodrine recently increased after it was threatened with a licence withdrawal in the USA. The reason cited was a failure of the manufacturing drug companies to provide previously agreed post-market studies. Conversely, midodrine has never received a licence from the UK regulatory authorities.
We provide a review of its current status and a brief description of our own experience with midodrine over the last 11 years.
Introduction
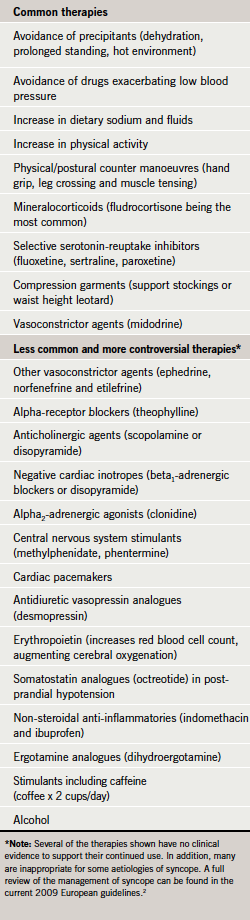
Epidemiological studies suggest that at least 40% of the population will experience at least one episode of syncope in their lifetime.1 The list of potential therapies available to treat hypotension and reduce the likelihood of syncope is extensive (table 1).
Midodrine is a potent α1 adrenergic agonist that has found widespread use in the treatment of low blood pressure and neurocardiogenic syncope. The active metabolite of midodrine, desglymidodrine, exerts vasopressor actions through activation of alpha-adrenergic receptors of the arteriolar and venous vasculature. Its effects are largely to raise both supine and, more particularly, standing blood pressure through a vasoconstrictor effect to increase peripheral vascular resistance. Although not widely used, midodrine currently ranks ninth in the Medicines and Healthcare products Regulatory Agency (MHRA) list of the top 10 unlicensed medicines imported into the UK in 2010.3
Role in treating hypotension
Hypotension, both postural (acute) and chronic has many aetiologies which can usually be addressed by the top five interventions shown in table 1. While infrequently used as first-line therapy, midodrine has established a role in patients who are unresponsive to more conservative manoeuvres (increased salt and fluid intake and physical counter-pressure techniques) and for patients who do not respond to treatment with the mineralocorticoid fludrocortisone.4 Midodrine has a short half-life of around three hours, which should necessitate a three times daily regimen. In practice, a proportion of patients seem adequately treated when it is used twice daily.
The most serious potential adverse effect from this drug is supine hypertension. One precaution to help prevent this is to advise patients not to take their last dose of the day later than early evening. Other potential side effects include urinary urge, retention and frequency, in addition to visual disturbances and headaches. Less serious are dermatological symptoms of pruritis (particularly of the scalp), ‘creeping skin’ sensation, piloerection (‘goosebumps’), parasthesae and chills. Aside from significant supine hypertension, additional contraindications to midodrine include severe organic heart disease, acute renal disease, urinary retention, phaeochromocytoma and thyrotoxicosis.
Current licence agreements
In the UK, midodrine occupies a unique position in the pharmacologic treatment of hypotension by remaining an unlicensed drug. Despite widespread use, no information appears in the latest British National Formulary, even with the suffix, as for some medicines, of being a recognised unlicensed use.5 In prescribing such products, the General Medical Council states “one should be satisfied that an alternative licensed product would not meet the patients needs and that there is sufficient evidence base or experience to demonstrate its safety and efficacy.” The prescribing physician bears the responsibility for the use of an unlicensed medicine and for overseeing the patient’s care, including monitoring and any follow-up treatment. The patient’s notes should indicate the medicine prescribed and, when it does not follow common practice, the reasons for using this product.
In effect, this is not dissimilar to the use of ‘licensed’ medicines when used ‘off-label’, as for example the use of selective serotonin-reuptake inhibitors (SSRIs) for vasovagal syncope. Many health trusts have assembled approved lists for either ‘unlicensed medicines’ or ‘unlicensed use of licensed medicines’, based on published evidence. The latter is common in paediatric prescribing, with evidence extrapolated from randomised-controlled trials in adults or ‘established’ clinical practice.
In the USA, midodrine was approved by the US Food and Drug Administration (FDA) agency in 1996, under the FDA’s accelerated approval regulations for drugs that treat life-threatening diseases. Seventy-nine of the 90 drugs approved through this process were for the treatment of cancer, human immunodeficiency/AIDS or the inhalation of anthrax.6 Approval for midodrine was granted on the basis that clinical trials be conducted to demonstrate improvement in the surrogate end point of blood pressure. The required post-market studies were to verify the clinical benefit of the drug; for example, in terms of improving a patient’s ability to perform day-to-day activities and safety.7 In August of 2010, the FDA threatened to withdraw the licence for midodrine, on the basis that post-approval studies to confirm the clinical benefit of the drug had not been performed.8 This represented a landmark step, being the FDA’s first attempt to remove a marketed medication on these grounds.7
Following intense pressure from patients and doctors, the FDA relented on their proposed action on the 6th September 2010, to allow time for the necessary data to be collected and certain ‘legal issues’ to be sorted out.9
Indications for the use of midodrine
Because midodrine can cause marked elevation in supine blood pressure (both systolic and diastolic), it is recommended that its use be restricted to patients whose lives are considerably impaired despite all standard clinical therapies. Vasovagal syncope is the most common abnormal response to upright posture and usually responds to education, increased dietary salt and fluids. Drug therapy becomes necessary when syncope is associated with physical injury, occupational hazard and a lack of warning. In vasovagal syncope, no convincing data exist to support the use of one drug over another as first-line therapy.1 Placebo-controlled studies provide limited evidence for the use of midodrine and SSRIs. Beta blockers are not now recommended to treat reflex-induced syncope.2 Permanent cardiac pacing is rarely needed and randomised trials do not support its use.10
Midodrine has been prescribed for various aetiologies of symptomatic hypotension. These comprise neurocardiogenic syncope,4,11 including vasovagal syncope,12,13 orthostatic hypotension in the elderly,14,15 autonomic nervous system dysfunction, haemodialysis-induced hypotension,16 spinal cord injury,17,18 infiltrative protein deposition disorders (e.g. amyloidosis),19 neurodegenerative diseases (e.g. Parkinson’s disease), through to a potential use in post-space-flight orthostatic hypotension.20 Perhaps more controversially, midodrine has recently been used successfully in advanced heart failure to raise the blood pressure sufficiently to prescribe disease-modifying therapies.21
Clinical trials and reviews of midodrine
In neurocardiogenic syncope, a baroreceptor-mediated reflex response results in hypotension and bradycardia, which can occur with orthostatic stress. Randomised-controlled trials in patients with severe symptomatic neurocardiogenic syncope have shown that midodrine is substantially more effective at relieving symptoms than more conservative approaches with increased fluids, salt and counselling.22,23 In a double-blind, placebo-controlled, tilt study on 12 patients with neurally mediated syncope, patients received a nonpressor 5 mg dose of midodrine or placebo. A positive tilt-test result occurred in 67% of patients in the placebo arm and 17% of patients taking midodrine.24
A recent meta-analysis of alpha-adrenoceptor agonist use for the treatment of vasovagal syncope, identified six randomised-controlled trials and supported the use of midodrine; citing it as a particularly efficacious compound.25 In contrast, the European guidelines on the management of syncope (2009 version) conclude that chronic treatment with alpha agonists alone may be of little use in reflex syncope and long-term treatment cannot be advised for occasional symptoms.2 For orthostatic hypotension midodrine should be administered as adjunctive therapy with a level of evidence deemed at level IIa (defined as a weight of evidence/opinion in favour of usefulness/efficacy).2
Additional considerations
In the USA, midodrine remains the only drug approved for the treatment of orthostatic hypotension.26 In the UK, its position as second-line therapy after fludrocortisone, could indirectly be a consequence of cost. A 5 mg three times daily dosage of midodrine is around four-fold more expensive than therapy with the ‘volume expander’ fludrocortisone at a dose of 100 µg daily (£16 vs. £2.96 per month).5 The fact that midodrine is not licensed and has a small market (around 100,000 patients in the USA) probably accounts for the paucity of published data on its use. In some specific circumstances, notably pregnancy, the total experience amounts to only two reported cases.27 Now 14 years after midodrine’s initial approval, the original manufacturer (Shire Pharmaceuticals) reports only a half million dollars annually from sales. Such low level of reimbursement begs the question as to “who could, or indeed should (when not financially viable for the company to do so) bear the costs of performing the ‘required’ clinical studies?”
Audit of midodrine use in a west London district general hospital
In an audit of midodrine use, at our institution, we identified 28 patients from pharmacy records who received this drug over the last 11 years. Those under 50 years of age were predominantly female (71%), those over 50 predominantly male (67%). Thirty-two per cent of patients had a diagnosis of vasovagal syncope, 14% postural hypotension and 54% had underlying autonomic dysfunction. All patients received midodrine at a dose of between 5.0 and 47.5 mg per day (mean 17.7 mg). In addition, 20 patients received fludrocortisone and five received SSRIs. Midodrine was generally well tolerated and no patients experienced adverse events that might be ascribed to the drug. Of note, a significant proportion of these patients (37%) also suffered concomitantly from either anxiety (stress at work or during college exams) (n=3), depression (n=4) or vascular dementia (n=3). In eight patients, supine blood pressures were ≤80 mmHg. Of these, seven were elderly (mean age of 71 years) and suffered from postural hypotension, one patient was aged 37 and suffered from vasovagal syncope.
We accept that the number of patients in this audit was small, representing around one patient per 100,000/year at our hospital. These observations suggest midodrine provides benefit in elderly patients with orthostatic hypotension from a variety of causes. We illustrate this with one case study from this audit.
Case report
A 72-year-old woman presented with profound hypotension. Out-patient sitting systolic blood pressures were recorded as low as 50 mmHg in association with pre-syncopal symptomology. The patient had two hospital admissions with symptoms and was found to have an IgG-kappa monoclonal gammopathy with clinically associated peripheral neuropathy.28 Mild benefit to blood pressure was achieved with fludrocortisone at 100 µg daily but the blood pressure was still inadequate to complete daily activities. Midodrine was started at 5 mg twice daily and gradually titrated to a daily dosage of 15 mg three times daily. Periodic adjustment of this prescription, in addition to fludrocortisone, achieved a blood pressure of 135/72 mmHg. The patient continued on midodrine for a further 92 months. Regular follow-up indicated a reasonable quality of life before eventually succumbing to overt myeloma at the age of 80 years.
Conclusion
In a ‘real-world’ clinical scenario, the number of patients, in whom symptoms progress to the extent that requires the use of midodrine, are small. This may reflect the caution that surrounds the use of unlicensed medicines. Midodrine appears to be well tolerated with wide application for hypotensive episodes resulting from several different aetiologies.4,11-24 In occasional cases, as illustrated above, it can provide life-saving treatment for profound hypotension.
Given these features and its undoubted efficacy we would propose that midodrine might find an expanded role in treating hypotension.
Conflict of interest
None declared.
Key messages
- Midodrine is a potent adrenergic agonist with pressor effects
- Midodrine remains an unlicensed medicine in the UK
- Midodrine has wide application in the treatment of hypotension from a variety of causes
- The available data are probably still insufficient to prove the efficacy of midodrine in vasovagal syncope (a very common cause of syncope)
References
- Aydin MA, Salukhe TV, Wilke I, Willems S. Management and therapy of vasovagal syncope: a review. World J Cardiol 2010;2:308–15. http://dx.doi.org/10.4330/wjc.v2.i10.308
- Moya A, Sutton R, Ammirati F et al. Guidelines for the diagnosis and management of syncope (version 2009). The Task Force for the diagnosis and management of syncope of the European Society of Cardiology (ESC). Eur Heart J 2009;30:2631–71. http://dx.doi.org/10.1093/eurheartj/ehp298
- Mathews GP. Summary report for importation of unlicensed medicines 1 April 2010 – 30 June 2010. Available from: http://www.mhra.gov.uk/Howweregulate/Medicines/Importingandexportingmedicines/Importingunlicensedmedicines/index.htm [accessed 19th January 2011].
- Sra J, Maglio C, Biehl M et al. Efficacy of midodrine in neurocardiogenic syncope refractory to standard therapy. J Cardiovasc Electrophysiol 1997;8:42–6. http://dx.doi.org/10.1111/j.1540-8167.1997.tb00607.x
- Joint Formulary Committee. British National Formulary. London: BMJ Group and Pharmaceutical Press, 2010;60.
- Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA 2010;304:2172–3. http://dx.doi.org/10.1001/jama.2010.1695
- Kuehn BM. Midodrine action. JAMA 2010;304:1317. http://dx.doi.org/10.1001/jama.2010.1334
- US Food and Drug Administration. Midrodrine hydrochloride: FDA proposes withdrawal of low blood pressure drug. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm222640.htm [accessed 19 September 2010].
- US Food and Drug Administration. Midodrine update. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm225444.htm [accessed 19 September 2010].
- Vaddadi G, Corcoran SJ, Esler M. Management strategies for recurrent vasovagal syncope. Intern Med J 2010;40:554–60. http://dx.doi.org/10.1111/j.1445-5994.2010.02295.x
- Low PA, Gilden JL, Frerman R et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double blind multicentre study by the Midodrine Study Group. JAMA 1997;277:1046–51. http://dx.doi.org/10.1001/jama.1997.03540370036033
- Kuriachan V, Sheldon RS, Platonov M. Evidence-based treatment for vasovagal syncope. Heart Rhythm 2008;5:1609–14. http://dx.doi.org/10.1016/j.hrthm.2008.08.023
- Quingou Z, Junbao D, Chaoshu T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J Pediatr 2006;149:777–80. http://dx.doi.org/10.1016/j.jpeds.2006.07.031
- Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med 2007;120:841–7. http://dx.doi.org/10.1016/j.amjmed.2007.02.023
- Paling D, Vilches-Moraga A, Akram Q et al. Midodrine hydrochloride is safe and effective in older people with neurocardiogenic syncope. J Am Geriat Soc 2010;58:2026–7. http://dx.doi.org/10.1111/j.1532-5415.2010.03069.x
- Perazella MA. Efficacy and safety of midodrine in the treatment of dialysis-associated hypotension. Expert Opin Drug Saf 2003;2:37–47. http://dx.doi.org/10.1517/14740338.2.1.37
- Barber DB, Rogers SJ, Fredrickson MD et al. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal Cord 2000;38:109–11. http://dx.doi.org/10.1038/sj.sc.3100959
- Wecht JM, Rosado-Rivera D, Handrakis JP, Radulovic M, Bauman WA. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehab 2010;91:1429–35. http://dx.doi.org/10.1016/j.apmr.2010.06.017
- Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112:2047–60. http://dx.doi.org/10.1161/CIRCULATIONAHA.104.489187
- Piwinski SE, Jankovic J, McElligot MA. A comparison of post space flight orthostatic intolerance to vasovagal syncope and autonomic failure and the potential use of the alpha-agonist for these conditions. J Clin Pharmacol 1994;34:466–71.
- Zakir RM, Folefack A, Saric M, Berkowitz RL. The use of midodrine in patients with advanced heart failure. Congest Heart Fail 2009;15:108–11. http://dx.doi.org/10.1111/j.1751-7133.2008.00042.x
- Ward CR, Gray JC, Gilroy JJ et al. Midodrine: a role in the management of neurocardiogenic syncope. Heart 1998;79:45–9.
- Perez-Lugones A, Schweikert R, Pavia S et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001;12:935–8. http://dx.doi.org/10.1046/j.1540-8167.2001.00935.x
- Kaufmann H, Saadia D, Voustianiouk A. Midodrine in neurally mediated syncope: a double-blind, randomized, cross-over study. Ann Neurol 2002;52:342–5. http://dx.doi.org/10.1002/ana.10293
- Liao Y, Li X, Zhang Y et al. Alpha-adrenoceptor agonists for the treatment of vasovagal syncope: a meta-analysis of worldwide published data. Acta Paediatr 2009;98:
1194–200. http://dx.doi.org/10.
1111/j.1651-2227.2009.01289.x - Lagi A, Spini S. Clinostatic hypertension and orthostatic hypotension. Clin Cardiol 2010;33:E10–E15. http://dx.doi.org/10.1002/clc.20722
- Glatter KA, Tuteja D, Chiamvimonvat N et al. Pregnancy in postural orthostatic tachycardia syndrome. Pacing Clin Electrophysiol 2005;28:591–3. http://dx.doi.org/10.1111/j.1540-8159.2005.50026.x
- Latov N. Pathogenesis and
therapy of neuropathies associated with monoclonal gammopathies. Ann Neurol 1995;37(suppl 1):
S32–S42. http://dx.doi.org/10.
1002/ana.410370705
