A 26-year-old man presented with T4 adenocarcinoma of sigmoid colon, which was initially treated with a covering ileostomy and neoadjuvant chemotherapy with oxaliplatin and infusional 5-fluorouracil delivered through a right subclavian Hickman line. While receiving chemotherapy, he developed a massive right atrial thrombus, adherent to the inferior venacaval opening and the adjoining right atrial wall, mimicking a metastatic deposit, which was removed surgically on cardiopulmonary bypass. The patient subsequently underwent successful high anterior resection of the sigmoid cancer followed by adjuvant chemotherapy with oxaliplatin and capecitabine. The unusual features of this patient’s presentation include the extremely rapid growth of thrombus despite aggressive anticoagulation, the unusual site of thrombus on the inferior vena caval opening rather than around the Hickman line, and possible facilitation of thrombus formation by chemotherapeutic agents. We also discuss the diagnostic and therapeutic dilemmas in a patient with a concurrent malignancy and a right atrial mass.
Introduction
Cardiac metastases from a colorectal carcinoma are rare. Presence of a mass in an unusual location in the heart in a young man with a galloping rate of growth, however, raises such a spectre. Decision algorithms revolve around the need to prevent a sudden catastrophic venous obstruction, risk of massive pulmonary embolisation, importance of ruling out metastatic disease to guide management of the primary malignancy, balancing the conflicting needs of early treatment of primary malignancy and the risks of doing this in the presence of an undiagnosed massive right atrial mass, and, finally, the conduct of the cardiac operation itself with infiltration of inferior vena cava and nearly whole of right atrium with organising thrombus.
Case presentation
A 26-year-old man presented with a three-day history of rectal bleeding, colicky abdominal pain and vomiting, and abdominal signs suggestive of a localised sigmoid colonic perforation, confirmed on a computed tomography (CT) scan. He was found to have a fungating, partially obstructing and circumferential lesion at the rectosigmoid junction 20 cm from the anal verge on sigmoidoscopy, which was diagnosed to be an adenocarcinoma on histology. A staging CT scan of abdomen and chest revealed a locally advanced and locally perforated distal sigmoid adenocarcinoma with retroperitoneal invasion. He underwent a loop ileostomy to relieve his abdominal symptoms, followed by five cycles of neoadjuvant OxMdG (oxaliplatin/modified de Gramont) chemotherapy with oxaliplatin and 5-fluorouracil (5-FU), via a Hickman line (figure 1), the sixth cycle having to be omitted due to neutropenia.
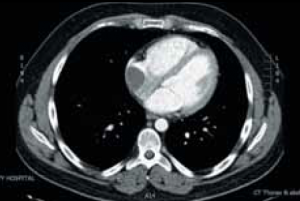
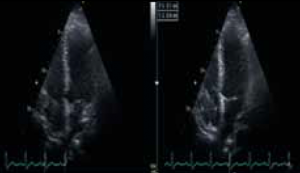
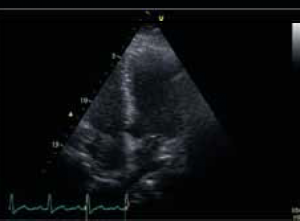
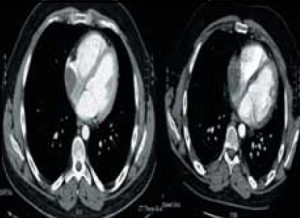
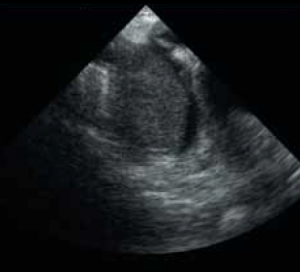
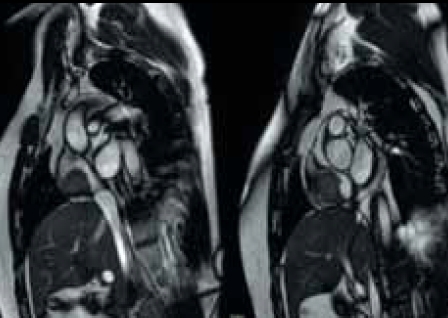
During the course of his chemotherapy, he was noted to have a 3.5 cm mass at the junction of inferior vena cava with right atrium on surveillance CT scan (figure 2). An echocardiogram confirmed an echo dense mass in the right atrium, discrete from the mobile Hickman line tip (figure 3), with no other valvular or ventricular pathology. Despite anticoagulation with heparin and warfarin, the right atrial mass continued to rapidly increase in size, as evidenced by echocardiogram (figure 4), CT scan (figure 5) and magnetic resonance imaging (MRI) scan (figure 6). MRI suggested a rapidly enlarging filling defect with minor heterogeneity in signal characteristics, with considerable vascularity under dynamic transit of gadolinium images, with no invasion of right atrial free wall (figure 6).
Differential diagnoses of thrombus, myxoma, metastasis from colorectal primary or other benign or malignant primary tumour of the heart were considered, but the actual aetiology of the mass was not considered to significantly change management at that stage. At the multi-disciplinary meeting, the risk of embolisation or obstruction to the tricuspid valve or inferior vena cava was considered a greater risk than the delay in removal of the primary tumour or the indeterminate effects of cardiopulmonary bypass on tumour biology or spread. Clinically, the patient was getting short of breath at minimal exertion, jugular venous pressure was 7 cm above sternal angle, heart rate was 80 beats per minute, regular, and blood pressure 110/76 mmHg. There were no cardiac murmurs or adventitious heart sounds. The patient’s pre-operative coagulation profile was normal.
It was elected to first perform excision of the right inferior venacavo-atrial mass under cardiopulmonary bypass with or without circulatory arrest, as indicated, followed by removal of the primary sigmoid tumour. On table transoesophageal echocardiography (TOE) confirmed further enlargement of the mass to involve three fifths of the right atrium and the inferior venacaval opening into the right atrium, without any plane of separation between the right atrium and inferior venacaval opening on one hand and the mass on the other (figure 7).
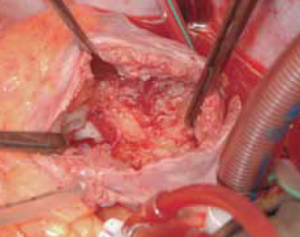
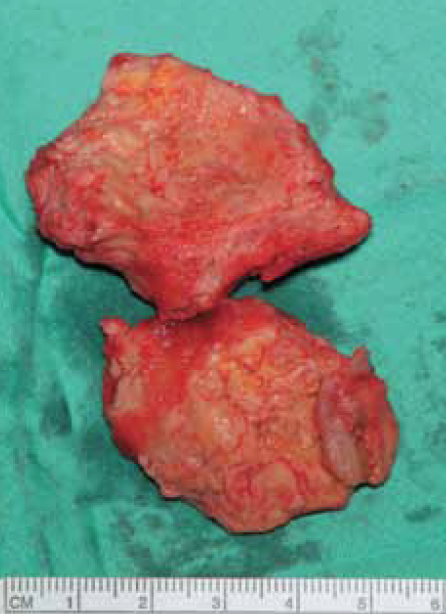
After median sternotomy, cardiopulmonary bypass was instituted with aortic, superior vena cava and left femoral venous cannulations. The aorta was cross clamped, heart arrested with 1.2 litre cold blood cardioplegia and right atrium opened by an oblique atriotomy. There was a large 5 cm x 7 cm fibrotic mass filling more than three fifths of the right atrium and densely adherent to the lower three fifths of the right atrium, including the mouth of the inferior vena cava (figure 8). The superior vena cava opening into the right atrium and, indeed, the Hickman line itself were entirely free of thrombus. The mass was dissected out from the right atrium and inferior vena cava by sharp dissection and removed along with the Hickman line. Macroscopically, it was difficult to differentiate the mass from a malignant tumour due to its hard consistency and tissue invasion, which required some atrial muscle to be included with the resection (figure 9). Histopathology of the mass, however, revealed an organising thrombus and no evidence of malignancy. The patient made a satisfactory postoperative recovery and was discharged home after 12 days. Postoperative coagulation studies revealed no abnormality.
Two months later, the patient underwent sigmoid colectomy for adenocarcinoma. The postoperative tumour classification was: ypT4, ypN1, V1, R1; Dukes C1. The patient underwent four cycles of adjuvant chemotherapy with oxaliplatin and capecitabine followed by reversal of ileostomy. He is free of all cardiac symptoms and is under regular tumour surveillance.
Discussion
Endocardial injury, stasis or turbulence of blood flow and hypercoagulability of blood are the three classical factors that predispose, in general, to intracardiac thrombosis.1,2
Right atrial thrombosis has been described with atrial fibrillation,3 constrictive pericarditis with tricuspid thrombotic obstruction4 and nonspecific pericarditis,5 restrictive cardiomyopathy6 and after Fontan operation.7,8 Genetic causes like methylenetetrahydrofolate (MTHF) reductase gene polymorphism,9 hypercoagulability characterised by V Leiden gene, thrombophilia genes and high serum homocysteine,10 myeloid leukaemia,11 heparin-induced thrombocytopenia,12,13 lung cancer14 and amoebic liver abscess15 have all been reported with right atrial thrombosis. Bi-atrial thrombosis with mitral stenosis and leg deep-vein thrombosis treated with mitral valve replacement and bi-atrial thrombectomy has been reported.16 On the other hand, there are reports of bi-atrial thrombosis across a patent foramen ovale, with resolution following anticoagulation.17 Some of the right atrial thrombi might be entrapped ‘in transit’ across a patent foramen ovale.10,18
However, the most common causes of right atrial thrombosis relate to the host of artificial devices inserted into the right atrium for a variety of indications. These include central venous catheters and Hickman lines for long-term use for antibiotics, hyperalimentation19 and chemotherapy,20,21 pulmonary artery catheters,19 permanent pacemaker22 and defibrillator leads,23 umbilical catheters,24 dialysis catheters25 and port-a-caths,26 extracorporeal membrane oxygenation (ECMO) catheters19 and ventriculo-atrial shunts for hydrocephalus27 or peritoneovenous shunts for intractable recurrent malignant ascites.28 Some of the device-related thrombosis has a septic component to it.29,30 Yang et al. reviewed the available literature on right atrial thrombosis in 2010 and identified 122 cases: 91% of patients had central venous catheters, 40.8% were prematurely born, 27.2% were post-cardiac surgery patients, 19.2% had underlying malignancies and 45.6% of patients received intravenous hyperalimentation.31
There were a number of substrates, in our patient, promoting thrombosis including long-standing presence of a Hickman line in the right atrium, the possibility of right atrial endocardial injury with the Hickman line, presence of systemic cancer and presumed hypercoagulable state secondary to this, although coagulation profile was normal, and the local thrombogenic effects of the chemotherapeutic agents on the right atrial endocardium. The adherence of the thrombus to the inferior wall of the right atrium, close to the inferior vena cava, right opposite the Hickman line, rather than to the Hickman line, which was entirely free of thrombus, heightens the suspicion of the role of the chemotherapeutic agents. Oxaliplatin toxicity includes sensory peripheral neuropathy, gastrointestinal disturbances, ototoxicity and myelosuppression. 5-FU can cause myelosuppression, mucositis, a rare cerebellar syndrome or a desquamative hand–foot syndrome. Although neither of these two agents is specifically known to cause coagulation abnormalities, they can cause thrombosis by direct endothelial injury as could have happened when the inferior wall of the atrium, being directly opposite the mouth of the Hickman line, was exposed to the stream of chemotherapeutic agents from the Hickman catheter.
Benign tumours and primary and secondary malignant tumours of the right atrium can masquerade as atrial thrombosis. Approximately 75% of primary cardiac tumours are benign. Approximately 50% of benign tumours are myxomas, and 10–20% of all myxomas arise from the right atrium. Other benign tumours that can arise from the right atrium are lipomas, lipomatous hypertrophy of interatrial septum, papillary fibroelastoma of tricuspid valve and hemangioma. A quarter of all primary cardiac tumours are malignant, and angiosarcomas, which have a predilection for the right heart, constitute 33% of all malignant tumours. The right atrium may also be involved by malignant mesothelioma or fibrosarcoma. Secondary tumours of the heart are more common than primary tumours and it is estimated that 10% of tumours eventually reach the heart or pericardium.32 The most common cancers responsible for cardiac metastases are leukaemia, melanoma, lung carcinoma, sarcoma, breast carcinoma, oesophageal, ovarian and kidney cancers. The usual means of spread to the heart are haematogenous, lymphatic, through direct extension and sub-diaphragmatic. Renal, hepatic, adrenal and uterine malignancies spread into the right atrium through the inferior vena cava. As many as 10% of all renal malignancies involve the inferior vena cava and 40% of these invade the right atrium.32
The extremely rapid rate of growth of the right atrial mass in our patient favoured the diagnosis of thrombus rather than a myxoma, although a rapidly growing metastatic tumour could not be entirely ruled out. Although no absolute figures are available, thrombi are much more common than either benign or malignant right atrial tumours.
The peak incidence of colorectal carcinoma is at age 60 to 70 years. Fewer than 20% occur in ages less than 50 years. When colorectal carcinoma is found in a young person, ulcerative colitis or one of the polyposis syndromes must be suspected. Morphologically, the distal colonic lesions tend to be annular, encircling lesions that produce napkin ring constrictions of bowel in contrast to the fungating polypoid masses on the right side. All colorectal tumours spread by direct extension into adjacent structures and by metastases through lymphatics and blood stream to the regional lymph nodes, liver, lungs, bones, peritoneal cavity, brain and others.33 In a large series of 2,435 patients with metastatic heart disease, Perry et al. reported colonic cancer accounted for 3.7% patients only.32 De la Fouchardiere reported a fortuitous discovery of a right ventricular colorectal secondary.34 Lattuada et al. reported pericardial metastases in a resected colonic adenocarcinoma35 and Chen et al. reported hemopericardium and cardiac tamponade secondary to pericardial metastases from colonic adenocarcinoma palliated by pericardiotomy.33
Colorectal cancer can co-exist with concurrent cardiovascular disease. Davydov reported 14 patients with different stages of colorectal cancer with concomitant coronary artery disease, valve disease, aortic aneurysm or a combination of the above, who underwent simultaneous operations (3) and staged operations (11) with 7.5% early mortality and 21.4% mortality during follow-up period from tumour relapse.36
Management of right atrial thrombosis is determined by symptoms, morphology of thrombus including size, shape, mobility and nature of thrombus, any associated infection or diagnostic uncertainty, risk of pulmonary embolism, tricuspid obstruction, etc. Removal of precipitating causes including central venous lines or pacemaker or defibrillator leads, medical treatment including anticoagulation and/or thrombolysis, thrombectomy under cardiopulmonary bypass with or without circulatory arrest and with or without selective cerebral perfusion, can all be employed depending on the size, chronicity and site of thrombosis.30,31 Large thrombi, chronic organised thrombi, thrombi associated with infection, thrombi whose aetiology is not clear and where metastatic malignancy is a possibility, or thrombi likely to embolise to the pulmonary circulation due to their sheer size, mobility and morphology are best treated surgically.31 Involvement of superior or inferior vena cava by thrombus usually requires thrombectomy under low flows or circulatory arrest with37 or without selective antegrade cerebral perfusion.38 Unusually, more complex procedures may be required. Dittrich et al. described surgical thrombectomy and re-insertion of superior vena cava in the right atrial appendage in the beating heart in a critically ill infant.39 Off-pump right atrial thrombectomy with a brief period of inflow occlusion has been described for heparin-induced thrombocytopenia with thrombosis.13 Percutaneous endovascular mechanical thrombectomy has been described using inferior vena cava basket and transjugular insertion of inferior vena cava filter under fluoroscopic and TOE guidance.40
Our patient, while undergoing pre-operative chemotherapy with 5-FU and oxaliplatin for adenocarcinoma of sigmoid colon, developed a rapidly enlarging right atrial mass that came to occupy three fifths of the right atrial cavity and was adherent to the inferior wall of right atrium, inferior venacavo-atrial junction and proximal inferior vena cava. While the presence of a Hickman line in the right atrium raised the index of suspicion towards a thrombus, a coincidental myxoma or another benign tumour like lipoma, papillary fibroelastoma or hemangioma, a malignant tumour known to favour the right heart like angiosarcoma or even mesothelioma, and, finally, a metastasis from the primary tumour, although extremely rare, could not be completely ruled out. Involvement of the cavo-atrial junction and inferior vena cava further complicated the diagnosis. Involvement of the inferior vena cava and most of the right atrium mandated direct cannulations of femoral vein and superior vena cava for venous return during cardiopulmonary bypass. Invasion of the cavo-atrial junction and proximal inferior vena cava by the organised thrombus required sharp dissection of the thrombus from inferior venacaval and right atrial myocardium with periods of low flow not unlike the dissection that is needed when renal, hepatic, adrenal or uterine tumours sneak up the inferior vena cava into the right atrium. We believe co-existence of cancer, Hickman line and chemotherapeutic infusions predisposed the patient to thrombus formation. Whether there was any direct endocardial injury due to mechanical catheter trauma would be difficult to establish with certainty.
Conflict of interest
None declared.
Key messages
- Colorectal metastases involve the heart rarely; most such secondaries involve pericardium, although cardiac chamber involvement has been reported anecdotally
- The most common cause of right atrial thrombus in the current era is indwelling instrumentation
- General hypercoagulability associated with cancer and local toxic effects of chemotherapeutic agents on the atrial endocardium predispose to thrombosis
- Hickman line, 5-fluorouracil and oxaliplatin infusion and colorectal cancer all contributed in varying measures to the development of massive inferior venacavo-atrial thrombus
References
- Cotran RS, Kumar V, Robbins SL. Hemodynamic disorders and shock. In: Schoen FJ (ed.). Robbin’s pathologic basis of disease. Philadelphia: WB Saunders, 1994;105–06.
- Konkle BA, Schaffer AI. Hemostasis, thrombosis, fibrinolysis and cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E (eds.). Braunwald’s heart disease. Philadelphia: Elsevier Saunders, 2005;2075.
- Lanjewar DN, Ramraje S, Lanjewar SD. Right atrial appendage thrombus with atrial infarct in a case of thyrotoxicosis: an autopsy report. Indian J Pathol Microbiol 2010;53:538–40. http://dx.doi.org/10.4103/0377-4929.68295
- Priestley KA, Wallwork J, Schofield PM. Right atrial thrombus in constrictive pericarditis. Int J Cardiol 1992;37:256–8. http://dx.doi.org/10.1016/0167-5273(92)90217-Q
- Toda R, Yuda T, Nishida T, Toyohira H, Taira A. Right atrial mural thrombus associated with pericarditis. Ann Thorac Surg 1996;62:1505–06. http://dx.doi.org/10.1016/0003-4975(96)00399-2
- Turgut O, Yalta K, Yilmaz MB, Dizman R, Tandogan I. Free-floating biatrial thrombi with concomitant saddle pulmonary embolism. Int J Cardiol 2010;144:e11–e13. http://dx.doi.org/10.1016/j.ijcard.2008.12.035
- Masqura VX, Marini M, Portela F, Cao I. A complication of classical Fontan operation: giant right atrial thrombus and massive pulmonary thromboembolism. J Card Surg 2008;23:776–8. http://dx.doi.org/10.1111/j.1540-8191.2008.00656.x
- Hedrick M, Elkins RC, Knott-Craig CJ, Razook JD. Successful thrombectomy for thrombosis of the right side of the heart after the Fontan operation. Report of two cases and review of the literature. J Thorac Cardiovasc Surg 1993;105:297–301.
- Motovska Z, Widimsky P, Bilkova D, Penicka M. An embolus in the right atrium caught in the Chiari network and resistant to thrombolysis. J Thromb Thrombolysis 2010;30:114–18. http://dx.doi.org/10.1007/s11239-009-0403-6
- Eweda II, Samir S, Abbas O, El-Gohary GM, Nammas W. Right heart thrombus-in-transit with pulmonary embolism in a patient with primary hypercoagulable state. Cardiol J 2010;17:408–11.
- Nanjappa MC, Shankarappa RK, Kalpana SR, Bhat P, Moorthy N. Intracardiac thrombi in acute myeloid leukemia: an echocardiographic and autopsy correlation. Echocardiography 2010;27:E4–E8. http://dx.doi.org/10.1111/j.1540-8175.2009.01047.x
- Chun KR, Bansch D, Bauer R, Scneider C, Kuck KH. Massive right atrial thrombus due to heparin-induced thrombocytopenia type II. Herz 2008;33:464–5. http://dx.doi.org/10.1007/s00059-008-3165-6
- Morgan JA, Kherani AR, Vigilance DW, Cheema FH, Colletti NJ, Sahar DI. Off-pump right atrial thrombectomy for heparin-induced thrombocytopenia with thrombosis. Ann Thorac Surg 2003;76:615–17. http://dx.doi.org/10.1016/S0003-4975(03)00159-0
- Sabharwal P, Ruggles S, Gharagozloo F. Right atrial thrombus in a patient with stage IV carcinoma of the lung: is the surgical treatment the correct choice? J Cardiovasc Surg 1998;39:689–90.
- Ur Rehman Z, Rehman Alvi R, Bibi S. Hepatic vein and inferior vena cava thrombus extending into the right atrium: a rare complication of amoebic abscess. J Coll Physicians Surg Pak 2010;20:57–9.
- Tasdemir K, Sarli B, Kaya MG, Gunebakmaz O. Mobile biatrial thrombus in a patient with mitral stenosis under heparin infusion. Interact Cardiovasc Thorac Surg 2008;7:667–9. http://dx.doi.org/10.1510/icvts.2008.179093
- Genc C, Uzun M, Yiginer O, Baysan O. Thrombus entrapped in a patent foramen ovale, causing only vague symptoms. Turk Kardiyol Dern Ars 2009;37:563–5.
- Cakir C, Duygu H, Eren NK, Akyildiz ZI, Nazli C, Ergene O. Witnessing a rare event – thrombus seeking its route in the right atrium: “thrombus in transit”. J Cardiovasc Med 2008;9:1166–8. http://dx.doi.org/10.2459/JCM.0b013e328311eed8
- Kouchoukus NT, Blackstone EH, Doty DB, Hanley FL, Karp RB. Kirklin/Barrat-Boyes cardiac surgery. Philadelphia: Churchill Livingstone, 2003;1922.
- Kinova E, Zlatareva N, Goudev A. Right atrial thrombus from inferior vena cava after acute cardiotoxicity of 5-fluorouracil. Cardiol J 2008;15:284–5.
- Gadomski A, Jaranowska D, Ebinger K, Ozimek W, Brzewski M. Right atrial thrombus complicating chemotherapy by central venous catheterization in a child with Hodgkin’s disease. Wiadomosci Lekarskie 1998;51:266–9.
- Coleman DB, Debarr DM, Morales DL, Spotnitz HM. Pacemaker lead thrombosis with atrial thrombectomy and biventricular pacemaker and defibrillator insertion. Ann Thorac Surg 2004;78:e83–e84. http://dx.doi.org/10.1016/j.athoracsur.2003.09.115
- Kurisu S, Inoue I, Kawagoe T. Right atrial thrombosis after upgrading to a biventricular pacing/defibrillation system. Intern Med 2009;48:2101–04. http://dx.doi.org/10.2169/internalmedicine.48.2453
- Paupe A, Lenclen R, Blanc P, Chassevent J. Thrombosis of the right atrium after umbilical venous catheterization. Favourable outcome after early thrombectomy. Arch Fr Pediatr 1992;49:105–08.
- Ghani MK, Boccalandro F, Denktas AE, Barasch E. Right atrial thrombus formation associated with central venous catheters utilisation in hemodialysis patients. Intensive Care Med 2003;29:1829–32. http://dx.doi.org/10.1007/s00134-003-1907-8
- Vicol C, Nollert G, Mair H, Reichart B. Port-a-cath complicated by right atrial thrombus. Minimally invasive thrombectomy without cardiopulmonary bypass. Z Kardiol 2004;93:706–08.
- Kiefer M, Eymann R. Huge thrombosis as consequence of VA-shunts. Acta Neurochir 2010;106(suppl):95–99. http://dx.doi.org/10.1007/978-3-211-98811-4_16
- Mestres CA, de Lacy AM, Pomar JL. Massive right atrial and ventricular thrombosis after peritoneovenous shunting treated by thrombectomy and tricuspid valvectomy. Ann Thorac Surg 1987;44:205–06. http://dx.doi.org/10.1016/S0003-4975(10)62045-0
- Kentos A, Dufaye P, Jacobs F, De Smet JM, Serruys E, Thys JP. Candida albicans septic thrombosis of the right atrium is associated with a central venous catheter. Clin Infect Dis 1995;21:440–2. http://dx.doi.org/10.1093/clinids/21.2.440
- Sontiseni SP, White M, Singh S et al. Thrombectomy reduces the systemic complications in device-related right atrial septic thrombosis. Can J Cardiol 2009;25:e36–e41. http://dx.doi.org/10.1016/S0828-282X(09)70482-9
- Yang JY, Williams S, Brandao LR, Chan AK. Neonatal and childhood right atrial thrombosis; recognition and a risk-stratified treatment approach. Blood Coagul Fibrinolysis 2010;21:301–07. http://dx.doi.org/10.1097/MBC.0b013e3283333c7c
- Reardon MJ, Smythe WR. Cardiac neoplasms. In: Cohen LH, Edmunds LH Jr (eds.). Cardiac surgery in the adult. New York: McGraw Hill, 2003;1392.
- Chen JL, Huang TW, Hsu PS, Chao-Yang, Tsai CS. Cardiac tamponade as the initial presentation of metastatic adenocarcinoma from the colon: a case report. Heart Surg Forum 2007;10:e329–e330. http://dx.doi.org/10.1532/HSF98.20071068
- De la Fouchardiere C, Desseigne F, Orlandini F, de la Fouchardiere A, Negrier S. Cardiac metastases and colorectal cancer: a case study. Gastroenterol Clin Biol 2007;31:621–3. http://dx.doi.org/10.1016/S0399-8320(07)89443-7
- Lattuada S, Saggia C, Biaggi G, Santagostino A, Forti G. Pericardial metastasis in a long-surviving patient with sigmoid carcinoma. Tumori 2005;91:101–02.
- Davydov MI, Gerasimov SS, Shestopalova IM et al. Surgical treatment of patients with colorectal cancer and severe concurrent cardiovascular diseases. Khirurgiia 2008;8:10–17.
- Mazzola A, Gregorini R, Villani C et al. Cavoatrial tumour thrombectomy with systemic circulatory arrest and antegrade perfusion. Ann Thorac Surg 2007;83:1564–5. http://dx.doi.org/10.1016/j.athoracsur.2006.04.028
- Stewart JR, Carey JA, McDougal WS, Merrill WH, Koch MO, Bender HW Jr. Cavoatrial tumor thrombectomy using cardiopulmonary bypass without circulatory arrest. Ann Thorac Surg 1991;51:717–21. http://dx.doi.org/10.1016/0003-4975(91)90111-3
- Dittrich S, Schlensak C, Kecicioglu D. Successful thrombectomy of the superior vena cava thrombosis in a newborn after cardiopulmonary bypass surgery. Interact Cardiovasc Thorac Surg 2003;2:692–3. http://dx.doi.org/10.1016/S1569-9293(03)00209-3
- Beregi JP, Aumegeat V, Loubeyre C, Collet JM, Asseman P. Right atrial thrombi: percutaneous mechanical thrombectomy. Cardiovasc Intervent Radiol 1997;20:142–5. http://dx.doi.org/10.1007/s002709900123