Introduction
This supplement was developed by the authors following a one-day consensus meeting on acute heart failure, held at the Royal College of Physicians, London, 19th December 2012. The authors have a broad range of specialist expertise and experience related to the diagnosis, treatment and care of patients with acute heart failure, including emergency medicine, acute medicine, cardiology, care of the elderly, pharmacy and heart failure liaison nursing.
Chair
 Professor Martin Cowie
Professor Martin Cowie
Professor of Cardiology, Imperial College London (Royal Brompton Hospital)
Panel
Professor Derek Bell
Professor of Acute Medicine, Imperial College London (Chelsea and Westminster Hospital)
Mrs Jane Butler
Consultant Nurse, Barts Health NHS Trust, London Chest Hospital
Professor Henry Dargie
Emeritus Professor of Cardiology, University of Glasgow
Professor Alasdair Gray
Consultant in Emergency Medicine, Royal Infirmary of Edinburgh
Professor Theresa McDonagh
Professor of Heart Failure and Consultant Cardiologist, King’s College Hospital, London
Dr Hugh McIntyre
Consultant Physician Elderly Care, Conquest Hospital, Hastings
Professor Iain Squire
Professor of Cardiovascular Medicine, University of Leicester
Dr Jacqueline Taylor
Consultant Physician, Medicine for the Elderly, Glasgow Royal Infirmary
Ms Helen Williams
Consultant Pharmacist for Cardiovascular Disease, Southwark Health and Social Care, and South London Cardiac and Stroke Networks
Executive summary
 Heart failure currently affects about 900,000 people in the UK, and numbers are predicted to rise as a result of the changing demographics of the population.1 While recent efforts have focused on improving the outlook for patients with chronic heart failure (CHF), mortality and morbidity following admission for acute heart failure (AHF) are significant, with 11% of patients likely to die in hospital and 30% dying within a year.2
Heart failure currently affects about 900,000 people in the UK, and numbers are predicted to rise as a result of the changing demographics of the population.1 While recent efforts have focused on improving the outlook for patients with chronic heart failure (CHF), mortality and morbidity following admission for acute heart failure (AHF) are significant, with 11% of patients likely to die in hospital and 30% dying within a year.2
Growing evidence suggests that mortality can be reduced if patients are rapidly and accurately assessed in the Emergency Department, and admitted to a cardiology ward with expertise in the management of AHF – a situation mirroring the treatment of patients following acute myocardial infarction (AMI). But, all too often, delays in getting key diagnostic tests, inappropriate admission to general rather than cardiology wards, failure to initiate and maintain drug treatments with proven survival benefits, and failure to arrange effective follow-up via heart failure specialist nurse liaison services, result in suboptimal care.
As Clinical Commissioning Groups (CCGs) in England strive to deliver on the requirements of preventing premature deaths and helping patient recovery from acute illness,3 prioritising AHF would produce rapid gains for patients and significant financial savings for CCGs in terms of bed occupancy, readmission rates and need for long-term care.
The introduction of AHF patient pathways and care bundles in some acute hospitals and Trusts is already making a significant impact on clinical and economic outcomes. More widespread uptake of such initiatives could transform AHF from a Cinderella story into national success.
AHF call to action – why now?
AHF is a major cause of emergency hospital admission, with significant impact on National Health Service (NHS) resources. Yet, with no new treatments for 20 years and variable service provision around the UK resulting in suboptimal outcomes for many patients, AHF is widely seen as the ‘Cinderella’ of cardiology.
New initiatives aimed at improving AHF management, coupled with a promising pipeline of innovative treatments, should now facilitate a radical change in approach. Novel interventions and specialist services have already transformed the care of coronary artery disease and stroke patients, and the same can be achieved for those with AHF.
Based on admission data from approximately 90% of UK hospitals, the National Heart Failure Audit is demonstrating the impact of patient characteristics, use of evidence-based therapies and place of care on post-discharge AHF mortality and morbidity.2
New European Society of Cardiology (ESC) guidelines on acute and chronic heart failure bring together the latest evidence underpinning recommendations on diagnosis and treatment,4 and new guidance on AHF from the National Institute for Health and Clinical Excellence (NICE) is being developed.5
Heart failure is addressed by four evidence-based indicators in the Quality and Outcomes Framework (QOF),6 by 13 quality statements in NICE Quality Standard 9,7 and by four care standards in Healthcare Improvement for Scotland’s Clinical Standards for Heart Disease.8 In addition, the British Society for Heart Failure has produced guidance on provision of heart failure services in secondary and tertiary centres,9 and the ESC Heart Failure Association has published standards for delivering heart failure care.10
At a local level, a number of health bodies and hospitals have developed evidence-based care pathways for AHF.
With so much emphasis at all levels on the need to improve AHF management within the changing commissioning environment, now is the time to prioritise AHF, re-think service provision, and establish more effective care pathways.
AHF – what we know now
The National Heart Failure Audit now provides a detailed picture of AHF treatment in England and Wales, and there are comparable data for Scotland. In 2011/2012, hospitals in England and Wales submitted records to the National Heart Failure Audit on 41,635 heart failure admissions – about two thirds of all those recorded as hospital discharges by Hospital Episode Statistics.
These records showed that 48% of heart failure patients were treated in cardiology wards, 41% in general medicine, and 11% on other wards. Over half of male patients received treatment on cardiology wards compared with fewer than 40% of female patients. Median length of stay was nine days in cardiology wards and eight days in general medicine, but considerable variation in length of stay was found.
Overall, 11.1% of heart failure patients died in hospital (10.2% for men, 12.1% for women: p<0.001). In-hospital mortality was lower than that seen in the audit for 2010/11, and this fall is the first sign that local initiatives to improve AHF care can impact outcomes since the Audit was started in 2008/2009. However, mortality levels remain well above those seen in clinical trials and surveys in other countries, so there is clearly still room for improvement.
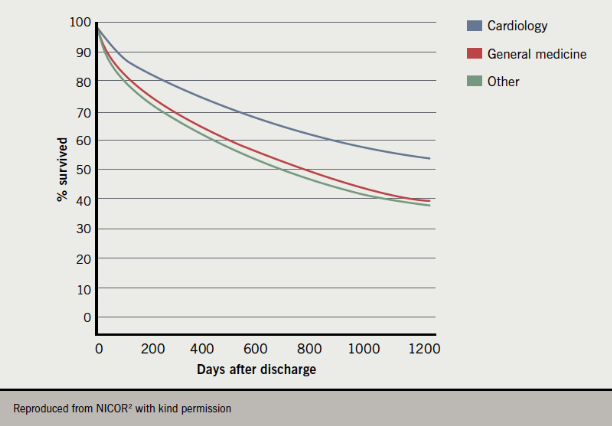
Mortality was lowest in cardiology wards (7.8%) and higher in general medical (13.2%) and other wards (17.4%). Even after adjusting for age, severity of heart failure and previous AMI, not being treated in a cardiology ward was associated with a worse outcome (hazard ratio [HR]=1.66 [1.52–1.81], p<0.001), although there may be other confounding factors.
The mortality advantage for cardiology ward treatment persisted for over three years post-discharge (figure 1).
(Click to enlarge any figure)
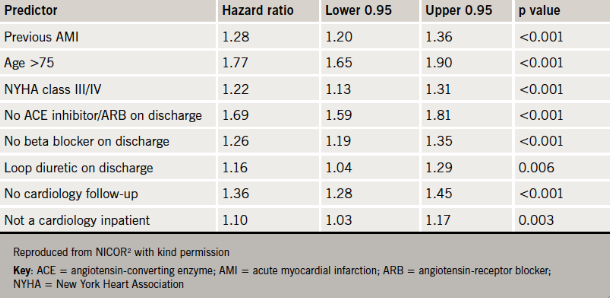
Other factors predictive of worse survival were left ventricular systolic dysfunction (LVSD), previous AMI, age >75, New York Heart Association (NYHA) class III/IV, absence of each of angiotensin-converting enzyme (ACE) inhibitor, angiotensin-receptor blocker (ARB) or beta blocker treatment on discharge, use of loop diuretic on discharge or a lack of cardiology follow-up (table 1).
Early accurate diagnosis is essential
Prompt diagnosis is key to ensuring that AHF patients are treated in the most appropriate place, which, as highlighted in the National Heart Failure Audit, is likely to be the cardiology ward for most patients. Like AMI, AHF needs specialist care.
For many patients with AHF, the diagnosis may not be immediately obvious or may be masked or complicated by co-morbidity. The presence of cognitive impairment, which is rising in prevalence, may further complicate the picture.
Patients presenting with AHF in the UK are generally elderly. The mean age of cases in the National Heart Failure Audit was 77.7 years (men 75.5, women 80.3). On first admission, 72% of patients were in NYHA class III/IV, and 45% had moderate or severe oedema. On readmission, 78% were in NYHA class III/IV and 52% had moderate or severe oedema. Sixty-five per cent of patients were diagnosed with LVSD, and this was more common in men than women (68% vs. 48%), and in those under 75 years.
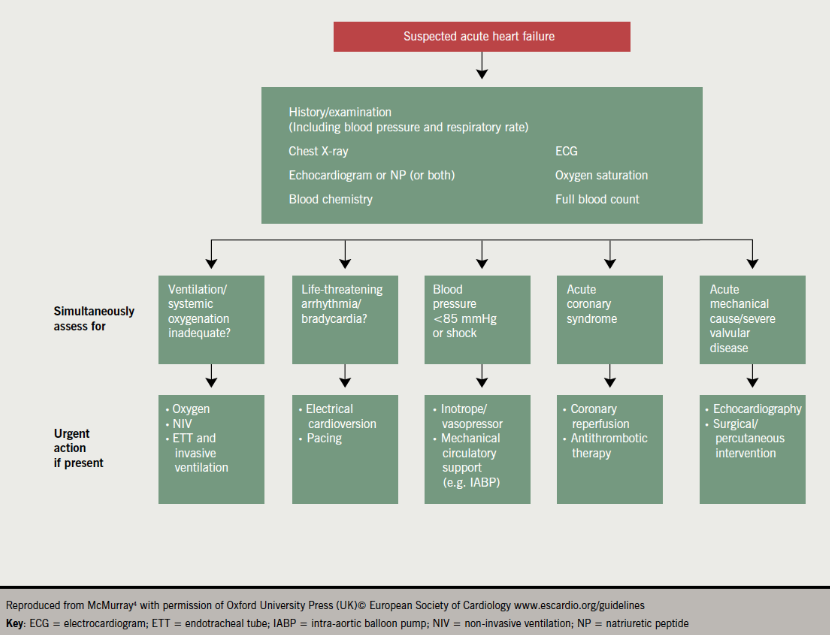
The key aspects of AHF diagnosis are summarised in figure 2, taken from the most recent ESC guidelines.4
B-type natriuretic peptide (BNP) acts as a flag for AHF, in the same way as troponin does for AMI. A normal level of BNP (<100 pg/ml) can help to exclude AHF. An initial BNP should be done within two to four hours and, for patients with a history of chronic heart failure (about two-thirds of AHF admissions), a comparison with pre-admission BNP levels may be more useful than a single measurement.
Confirmation of AHF requires evidence of cardiac dysfunction usually by an echocardiogram (echo), which, ideally, should be performed at presentation. Results of a recent echo (carried out within the previous six months) are acceptable, provided there has not been a clear history of a new cardiovascular event.
Diagnostically, the most challenging cases are patients with preserved ejection fraction (EF), but with a history suggestive of AHF. Such patients are typically older and female, and specialist input is essential to ensure accurate diagnosis.
Barriers to prompt diagnosis
 Many AHF patients enter the care pathway via the Emergency Department. But many Emergency Departments struggle to meet the four-hour assessment target, particularly during peak times and winter months.
Many AHF patients enter the care pathway via the Emergency Department. But many Emergency Departments struggle to meet the four-hour assessment target, particularly during peak times and winter months.
Such delays are of particular concern for AHF patients, as prompt diagnosis and treatment are essential. Those with known chronic heart failure tend to be more easily identified, but patients with new-onset AHF are particularly poorly served, especially when the diagnosis is challenging or there is co-morbidity.
Inaccurate diagnosis in the Emergency Department can result in patients being transferred to inappropriate wards, resulting in longer hospital stays, higher readmission rates and increased mortality. Timely diagnosis, leading to correct diagnostic ‘labelling’ and appropriate place of care, will lead to better outcomes for AHF patients.
The Glasgow experience
 In 2007, an audit across Glasgow hospitals revealed considerable variability in the organisation of care and an inconsistent approach to AHF. Overall, 90% of patients admitted with decompensated heart failure were moved to a second ward, and 24% were moved to a third ward. On one site in particular, there was limited specialist input (10%) and significant delays in echocardiography were common (mean delay to echo request – 8.4 days). During a five-week period, an estimated £52,000 was wasted in unnecessary bed occupation while patients waited for echo. In addition, there was suboptimal use of evidence-based therapy, and limited access to heart failure nurse and specialist follow-up.
In 2007, an audit across Glasgow hospitals revealed considerable variability in the organisation of care and an inconsistent approach to AHF. Overall, 90% of patients admitted with decompensated heart failure were moved to a second ward, and 24% were moved to a third ward. On one site in particular, there was limited specialist input (10%) and significant delays in echocardiography were common (mean delay to echo request – 8.4 days). During a five-week period, an estimated £52,000 was wasted in unnecessary bed occupation while patients waited for echo. In addition, there was suboptimal use of evidence-based therapy, and limited access to heart failure nurse and specialist follow-up.
After a subsequent merger of two major Glasgow hospitals, a later audit showed care pathways were highly dependent on whether or not patients had LVSD (about half of cases). While two thirds of LVSD patients were admitted directly to a cardiac ward, this occurred in less than half of non-LVSD patients. Those not admitted directly to the cardiac ward were admitted to the acute Medical Admissions Unit (MAU). From there, 42% of the LVSD patients were referred to the cardiac ward, compared with only 14% of the non-LVSD patients. The latter group were most likely to be referred to the Care of the Elderly or general medical wards.
The merger of hospitals allowed the development of a large cardiology ward and the streamlining of the AHF pathway. Subsequently, the mean wait for echo fell to four days, 90% of LVSD patients had an expert review (69% of non-LVSD patients), and three-quarters of patients who were eligible to see heart failure liaison nurses accessed this service. In-patient mortality was 9.5%, all-cause readmission at one month was 21%, and median length of stay fell from 12 days to 10 days – all improvements on the situation prior to the re-organisation. The success of this restructuring was due to a number of factors, including better coordination of care, a geographical focus, multi-disciplinary team (MDT) working and formal MDT meetings, and to the introduction of an early supported discharge initiative which enables carefully selected patients to be discharged earlier, without any adverse effect on readmission rate.
Patient pathway
Building on this initial success, a patient pathway has recently been developed for the Glasgow area which includes triage of patients in Acute Admissions Unit (AAU)/MAU according to BNP, echo and co-morbidities, so that the vast majority of AHF patients can be referred to cardiology wards where they will get specialist review and referral to a heart failure liaison nurse. This pathway enables frail elderly patients to be treated in Care of the Elderly wards, but with input from specialists, and includes triggers for additional cardiology review for patients who have had prolonged admissions or have had previous AHF admissions. The aim is to ensure rapid recovery, routine follow-up by heart failure liaison nurses, and early supported discharge into a primary care locally enhanced service or, if appropriate, into palliative care services.
The King’s experience
 The Heart Failure Unit at King’s College Hospital, London, has recently developed an Acute Heart Failure Pathway, so that patients are seen in the Acute Admissions Unit (AAU) rather than several days later in general medical wards. The service comprises:
The Heart Failure Unit at King’s College Hospital, London, has recently developed an Acute Heart Failure Pathway, so that patients are seen in the Acute Admissions Unit (AAU) rather than several days later in general medical wards. The service comprises:
- a daily consultant-led ward round by a cardiologist with an interest in heart failure for referrals in the AAU
- dedicated echo slots
- review by a hospital-based specialist heart failure nurse.
Patients who have most to benefit from cardiology care are transferred to Cardiology and a decision is made about those who would be better managed by the Care of the Elderly Service. All patients have a specialist cardiology opinion and access to further cardiology medical and nursing reviews during their inpatient stay. The Heart Failure Unit then arranges appropriate Cardiology and Heart Failure Nursing Specialist follow-up on discharge.
Preliminary results demonstrate better prescription rates of key drugs for all patients (admitted to Cardiology and General Medicine) than in the National Heart Failure Audit 2011/12, i.e. ACE inhibitor/ARB 92.4% vs. 84%, beta blockers 87% vs. 78% and a one-year mortality rate of 15.6% compared with the greater than 30% seen nationally.
The Hastings experience
The Hastings heart function service was established in 1998 with the appointment of one full-time acute heart failure nurse (AHFN). A fully integrated service, covering a population of approximately 180,000, was introduced following the appointment of two full-time community heart failure nurses (CHFN) in 2005.
The service enables:
- early identification of AHF patients by daily review of Medical Admission Unit (MAU) documentation by the AHFN. Automatic ‘flags’ from the clinical information system identify readmission of any patient already under the heart failure service
- specialist stratification of inpatient care according to diagnosis and severity, irrespective of ward and whether admission is for new-onset heart failure or for decompensation
- coordinated discharge planning by AHFN and CHFN with urgent follow-up, including echo at the rapid access heart function clinic within two weeks of discharge. All recently discharged patients are discussed at multi-disciplinary team meetings.
 The service is supported by weekly MDT meetings, bi-weekly ward round review (as part of the lead physician’s general medical round), and verbal, email and telephone contact between all team members (hospital, community, lead physician and consultant cardiologists).
The service is supported by weekly MDT meetings, bi-weekly ward round review (as part of the lead physician’s general medical round), and verbal, email and telephone contact between all team members (hospital, community, lead physician and consultant cardiologists).
In 2010, formal evaluation by the Heart Improvement Programme found the service was clinically and cost effective, resulting in a sustained reduction in admissions that more than offset the cost of delivery.12
Data on heart failure discharges, validated to July 2012, showed that the service achieved 100% compliance with all four care indicators: echo, ACE inhibitor/ARB at discharge in patients with systolic dysfunction, discharge instructions (activity level, diet, discharge medications, follow-up appointment, weight monitoring and advice if symptoms worsen), and advice on smoking cessation.
As part of the Enhancing Quality Programme, a substantive part-time discharge support nurse (DSN) has been appointed to provide pre-discharge advice and counselling for patients with a new diagnosis of heart failure, and structured follow-up in the community for 30 days post-discharge. Patients are risk stratified at discharge and formal Transfer of Care documentation allows structured handover to the CHFN.
The 30-day readmission rate for heart failure for the year to October 2012 was 5.5%. In 39 consecutive discharges in the three months after the DSN appointment, there were no deaths in the 30 days following admission, and one readmission for heart failure.
It is planned to establish designated heart failure beds within a subspecialist ward area, allowing daily input from the specialist team with a view to early supported discharge.
A pilot study is underway evaluating the use of BNP in the Emergency Department and MAU to further assist early patient identification and care stratification.
Currently, the MDT meeting covers all inpatients and all patients within 30 days of discharge. A ‘virtual ward’ is being developed to include patients stratified as high risk and those undergoing active intervention in the community (including the use of intravenous diuretics in the community).
Treatment – more than just symptom relief
AHF treatment is often started alongside the diagnostic work-up, and is based on symptom relief, improvement of haemodynamics, respiratory support if required, risk stratification and management of precipitating factors.
The goals of treatment are outlined in table 2.
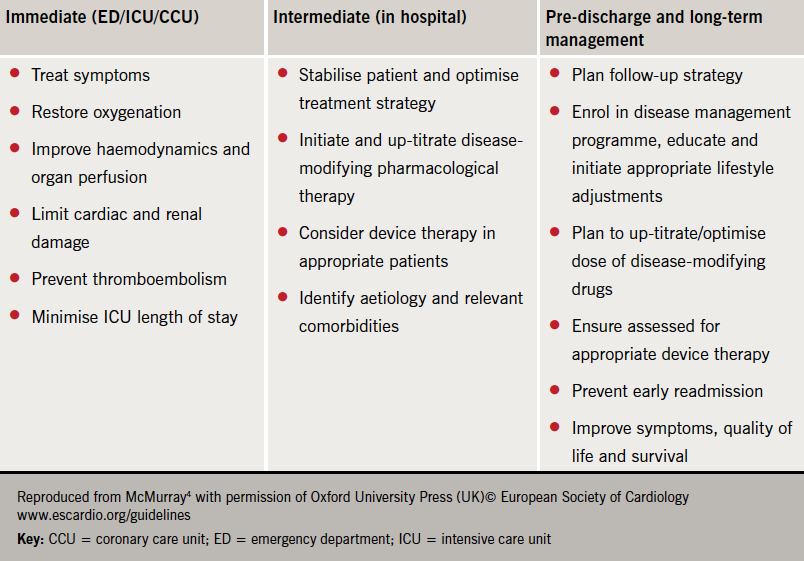
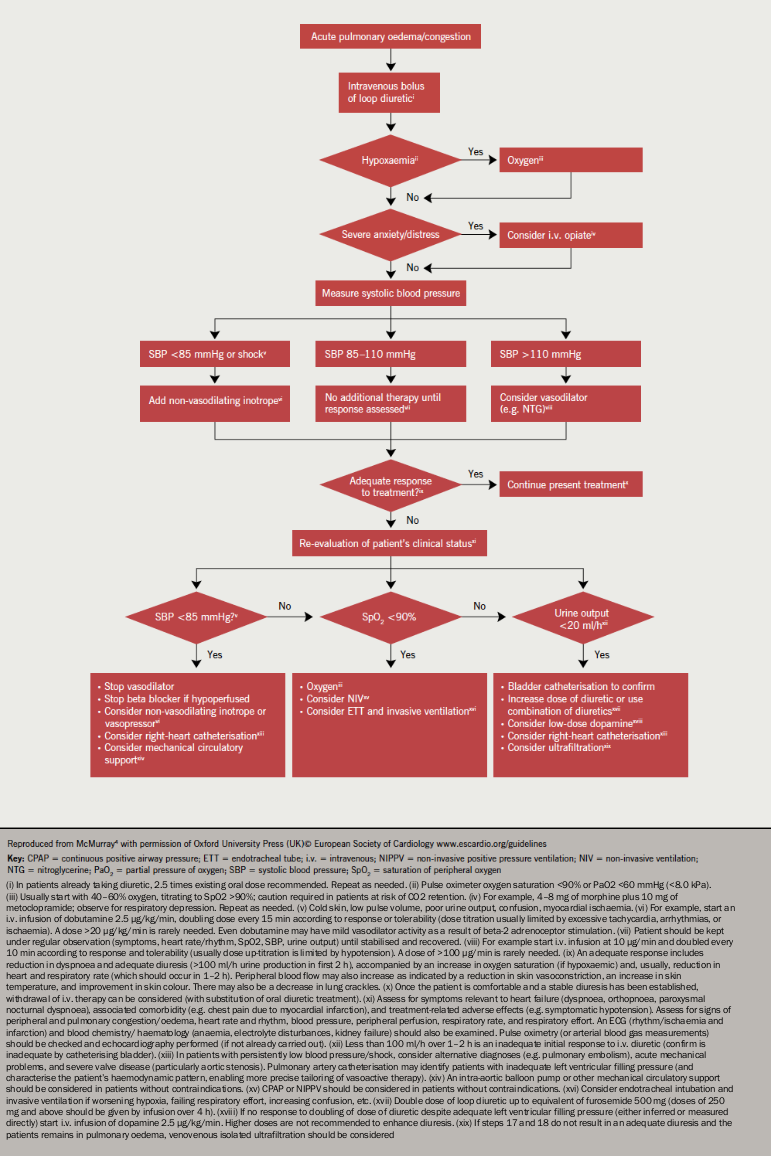
Acute management is focused on diuretics to relieve dyspnoea, oxygen to treat hypoxaemia and, where appropriate, opiates to relieve distress associated with dyspnoea (figure 3). Vasodilators are considered most useful for patients with hypertension and should be avoided in those with a systolic blood pressure <110 mmHg.2
It is important to address left ventricular (LV) dysfunction as early as possible – in the Emergency Department or the Acute Medicine Unit (AMU). In the absence of contraindications, patients with reduced EF who are not already using ACE inhibitors (or ARBs), beta blockers and mineralocorticoid receptor antagonists (MRAs) should be started on all these drugs, carefully and sequentially according to blood pressure and renal function, after their initial symptoms and clinical status are stabilised.4
Data from the National Heart Failure Audit demonstrate the importance of ACE inhibitor/ARB, beta blocker and MRA treatment for both short- and long-term survival.2 Patients with LVSD who were discharged on all three groups of drug were nearly twice as likely to be alive 12 months later compared with untreated patients, and this advantage persisted for at least three years after discharge (figure 4). In contrast, patients discharged on loop diuretics alone had a worse survival in the first year after discharge.
Audit data showed that patients with LVSD treated in cardiology units were twice as likely to be discharged on beta blockers as those treated in general wards, and also more likely to go home on ACE inhibitors/ARBs and MRAs.2
The value of rehabilitation programmes for patients with chronic heart failure is now recognised, and NICE Quality Standard 9 recommends that people with stable chronic heart failure and no precluding condition or device should be offered a supervised group exercise-based cardiac rehabilitation programme that includes education and psychological support following heart failure admission.7 Currently, only a small minority of patients are offered and attend such a programme.
![Figure 4. Three-year post-discharge survival by use of drugs (angiotensin-converting enzyme [ACE] inhibitor/angiotensin-receptor blocker [ARB], mineralocorticoid receptor antagonist [MRA], beta blocker)](https://bjcardio.co.uk/files/uploads/2013/03/Figure-41.png)
Barriers to effective treatment
Major problems are arising in both the acute management of AHF and after discharge.
In the acute phase, inappropriately aggressive treatment with intravenous diuretics and vasodilators can result in intravascular volume depletion and hypotension. Slow recognition of the severity of AHF in some patients may delay introduction of ventilatory or circulatory support, increasing the risk of cardiorespiratory arrest.
Failure to recognise the importance of identifying patients likely to benefit from administration of ACE inhibitors/ARBs, beta blockers and MRAs, and the need for continued treatment post-discharge, also leads to suboptimal treatment.
Nursing input into multi-disciplinary care has been limited almost exclusively to patients with chronic heart failure due to systolic LV dysfunction, with limited ‘pull in’ from acute admissions. As a result, many AHF patients are missing out on nurse-led interventions and entry into a disease management programme, which can improve long-term outcomes.11
Service realignment
AHF is primarily a cardiologic condition, so care should be provided primarily by cardiologists or those with a special interest and experience in heart failure. Involvement of such specialists should start in the Emergency Department, via agreed pathways for input prior to referral to a cardiac care unit (CCU), AMU or Care of the Elderly ward.
All too often, current services find emergency and acute physicians “pushing unsuccessfully at the doors” of cardiology services to get their AHF patients admitted. There is an urgent need to replace this with a ‘pull in’ approach, whereby cardiologists identify patients suitable for cardiologic assessment and input as soon as they enter the AHF pathway.
How can we encourage improvements in the AHF pathway?
Key drivers for change
- Benchmarking of local AHF management against national performance and standards, highlighted by the National Heart Failure Audit and other studies, to identify:
– lack of cardiology input at presentation in the Emergency Department and AMU
– delays in getting essential tests e.g. echo and BNP
– failure to assess, initiate and maintain patients on key treatments (e.g. beta blockers, ACE inhibitors/ARBs and MRAs)
– failure to admit patients to specialist areas (e.g. cardiology wards or wards with specialist cardiology input)
– lack of follow-up by specialist cardiology nurses
– high rates of inpatient mortality
– high rates of readmission.
- New ESC4 and other guidance on effective diagnosis and treatment of AHF (NICE guidance due in 2014).
- Good evidence base showing that place of care and AHF management impact on survival and hospital admissions.
- Clinical Commissioning Groups working to deliver on the domains of ‘Liberating the NHS’, i.e.:
– preventing people from dying prematurely
– enhancing the quality of life for people with long-term conditions
– helping people to recover from episodes of ill health or following injury
– ensuring people have a positive experience of care
– treating and caring for people in a safe environment and protecting them from avoidable harm.
- Updated QOF targets for heart failure for 2013/14:
– at time of going to press these have not been published. During 2012/13, QOF has had four HF indicators, with a maximum of 29 points, achieved if the practice has a HF register, there is high use of echo to confirm a diagnosis, with at least moderate use of ACE inhibitors or ARBs, and beta blockers, for those with heart failure and left ventricular dysfunction. 6 A rehabilitation referral indicator has been suggested but has not yet been adopted.
- NICE Quality Standards for Chronic Heart Failure relevant to AHF management, i.e.:
– measurement of serum natriuretic peptides
– multi-disciplinary heart failure team input
– treatment with ACE inhibitors/ARBs and beta blockers
– cardiac rehabilitation programme
– management plans for patients admitted to hospital
– hospital discharge and follow-up care coordination.
- NICE Clinical Commissioning Group Outcomes Indicator Set (CCGOIS):
– at time of going to press, this set of indicators had not been agreed, but may include 30-day and 12-month mortality, and 30-day readmission rate.
Key factors for success
 Introduction of AHF pathways: with input from all relevant specialists, i.e. emergency medicine, acute medicine, cardiology, care of the elderly
Introduction of AHF pathways: with input from all relevant specialists, i.e. emergency medicine, acute medicine, cardiology, care of the elderly- A 24/7 service: as early accurate diagnosis in the Emergency Department can impact on long-term outcomes, it is essential that expert assessment and care is always available at the patient’s point of entry into the AHF pathway
- Patient phenotyping: full assessment and risk stratification will ensure accurate tailoring of acute and long-term treatment, including management of precipitating factors
- Specialist treatment in the optimal place of care: for most patients this will be in cardiology wards, but specialist expertise in AHF also needs to be available for patients who are treated in acute medical or care of the elderly wards
- AHF education: this is important for all those involved in patient care, particularly in the Emergency Department
- Support from management teams – AHF clinical enthusiasts/champions need to convince management of the importance of prioritising AHF and to present evidence of potential to improve quality, patient experience of care, and resource utilisation, while maintaining income streams
Conflict of interest
Professor Derek Bell: none declared. Mrs Jane Butler has received speaker or advisory honoraria from Novartis, Pfizer, Servier and Takeda, and travel bursaries from Merck, Roche, Servier and Takeda. Professor Cowie has received research grants from Boston Scientific, Medtronic, St Jude Medical and Servier; he has received consultancy fees in the past two years from Bayer, GE Healthcare, Novartis, Pfizer, Roche Diagnostics and Servier. Professor Henry Dargie has participated in Data and Safety Monitoring Boards for clinical trials funded by Amgen, Boston Scientific, Celgene, GlaxoSmithKline, Merck, Novartis, Pfizer, Servier and Vifor, and in research funded by Alere, GlaxoSmithKline and Pfizer. Professor Alasdair Gray has received fees for advisory board work from Novartis. Professor Theresa McDonagh has received honoraria for participation in national and international advisory boards from Novartis. Dr Hugh McIntyre has received research grants, travel bursaries, speaker or advisory fees from Boehringer Ingelheim, Merck, Novartis, Pfizer, Roche, Servier and Takeda. Professor Iain Squire has undertaken advisory board work for Novartis AG and Servier, and worked as a national chief investigator for Novartis AG. Dr Jacqueline Taylor has received honoraria/consultancy fees from Novartis, Pfizer and Servier. Ms Helen Williams has received speaker fees and/or attended advisory boards for Pfizer and Servier.
References
- National Institute for Health and Clinical Excellence. Chronic heart failure. Management of chronic heart failure in adults in primary and secondary care. CG 108. London: NICE, August 2010. www.nice.org.uk/cg108
- National Institute for Cardiovascular Outcomes Research (NICOR). University College London. National Heart Failure Audit 2011/12 Annual Report. London: NICOR, 2012. http://www.ucl.ac.uk/nicor/audits/heartfailure/additionalfiles/pdfs/annualreports/annual12.pdf/
- Department of Health. Equity and excellence: Liberating the NHS. London: DoH, 2010.
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. http://dx.doi.org/10.1093/eurheartj/ehs104
- National Institute for Health and Clinical Excellence. Acute heart failure. Scope available from: http://guidance.nice.org.uk/CG/Wave0/608
- NHS Employers and the General Practice Committee of the British Medical Association. Quality and Outcomes Framework for 2012/13: guidance for PCOs and practices. London: BMA, February 2012. Available from: http://www.nhsemployers.org/Aboutus/Publications/Documents/QOF_2012-13.pdf
- National Institute for Health and Clinical Excellence. Chronic heart failure. Quality Standards QS9. London: NICE, June 2011. Available from: http://guidance.nice.org.uk/QS9
- Healthcare Improvement Scotland. Clinical standards for heart disease. Edinburgh: NHS Quality Improvement Scotland, April 2010. Available from: http://www.healthcareimprovementscotland.org/programmes/cardiovascular_
disease/heart_disease/heart_disease_standards.aspx - British Society for Heart Failure. Defining the specifics for the provision of heart failure services in secondary and tertiary care. Available from: http://www.bsh.org.uk/resources/guidelines/
- McDonagh TA, Blue L, Clark AL, et al.; European Society of Cardiology Heart Failure Association Committee on Patient Care. European Society of Cardiology Heart Failure Association standards for delivering heart failure care. Eur J Heart Fail 2011;13:235–41. http://dx.doi.org/10.1093/eurjhf/hfq221
- Roccaforte R, Demers C, Baldassarre F, et al. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Failure 2005;7:1133–44. http://dx.doi.org/10.1016/j.ejheart.2005.08.005
- McIntyre HF, Barrett J. Is integrated heart failure care cost effective? Circulation 2010;122:A20767.

