Atrial fibrillation (AF) is the most common cardiac dysrrhythmia. The evidence base and expert consensus opinion for management have been summarised in several international guidelines. Recent studies suggest a disparity between contemporary practice and perceived best practice.
An electronic questionnaire was constructed to capture details of patient demographics and current practice, including risk assessment for stroke and major bleeding. All patients >18 years with AF as a primary or secondary diagnosis admitted from midday on the 14th September 2011 to midday on the 15th September 2011, were included in the survey. Participating units were recruited from the Society for Acute Medicine registry, and provided with an electronic link and password to enter data for individual patient episodes.
The electronic questionnaire was completed for 149 patient episodes from 31 acute medical units (AMUs) across the UK. The typical patient with AF presenting to the AMU is older, has important medical comorbidities (sepsis in almost a third) and frequently presents out of hours. Initial management was digoxin alone in 22% and 23% had a documented stroke risk assessment, not in-keeping with current guidelines.
This relatively simple methodology yields valuable insight into the real world management of AF, providing an additional evidence base.
Introduction
Atrial fibrillation (AF) is the most common cardiac dysrrhythmia, whose sequelae include stroke, heart failure and poor quality of life.1 In parallel with an ageing population, the prevalence of AF is increasing, with persistent or permanent forms affecting 10–15% of the population over the age of 75 years.2-6
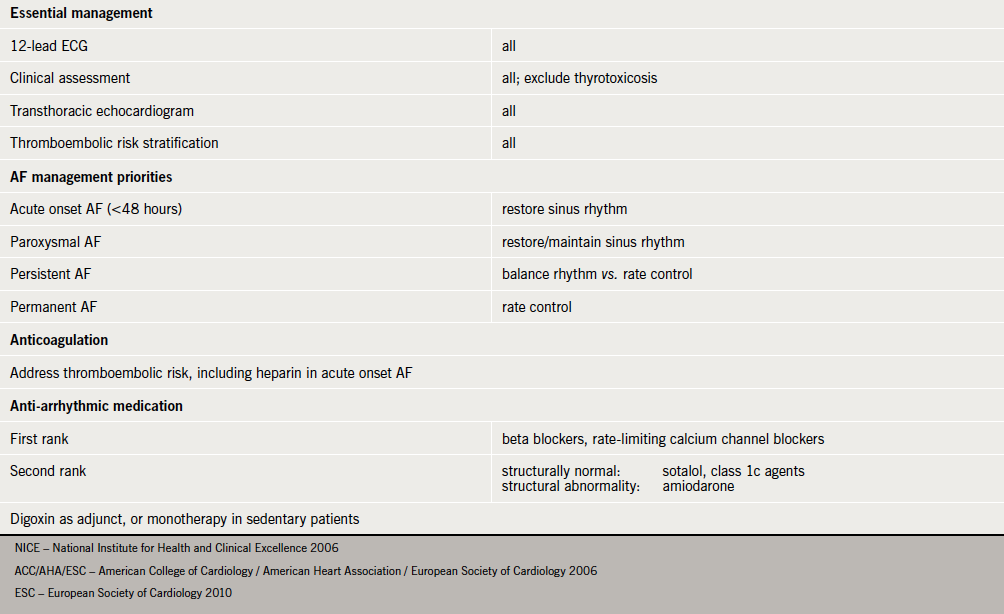
The effective management of AF has been a source of recurring debate, leading to the publication of combined American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC), and National Institute for Health and Clinical Excellence (NICE) guidelines in 2006.7,8 In addition to evidence-based strategies for rhythm and rate control, an important issue addressed in these guidelines was that of thrombotic risk assessment and management. This was further prioritised in the 2010 ESC document.9 Recent studies have suggested that both primary10 and secondary11 care may not follow guidelines for the management of AF.
Acute medical units (AMUs) represent the first
point of assessment and care for the majority of medical inpatients. Hospital episode statistic data suggest that, of the 5,287,032 emergency admissions in England from 1st April 2010 to 30th March 2011, 57,898 (1.1%) had a primary diagnosis of AF.
The number of AMUs affiliated with the Society of Acute Medicine (SAM) has steadily increased since its inception in the millennium, and now stands at 220.12 These units serve a substantive UK population base, creating considerable potential for collaborative research into current medical practice.
The Acute Medical Research Network of SAM, in association with the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) for Northwest London, commissioned a survey to examine contemporary management of AF within AMUs in the UK. We report the findings of this survey and its implications.
Methods
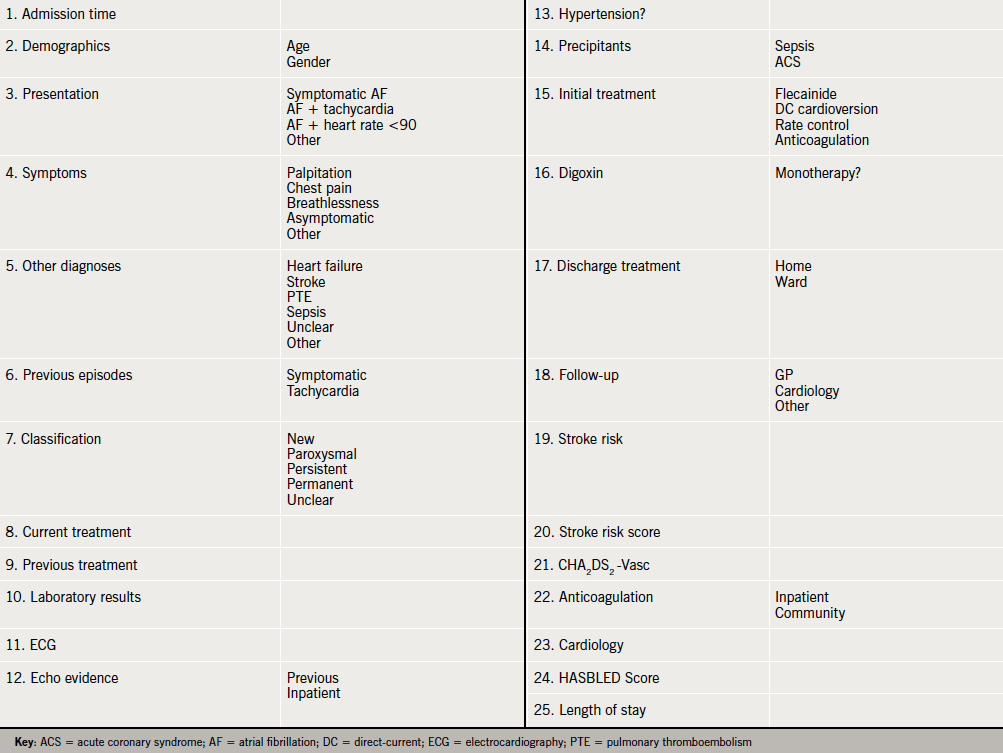
An electronic questionnaire was constructed with fields including risk assessment for stroke and major bleeding, citing widely used scoring systems (see Appendix 1).
All patients >18 years with AF as a primary or secondary diagnosis admitted over a specific 24-hour time period (midday on 14th September to midday on 15th September 2011) were included in the survey. Participating AMUs were provided with a link and password to enter data for each patient episode into the survey. Units were advised to nominate an individual to coordinate participation at a local level to prevent duplication of data entry.
Results
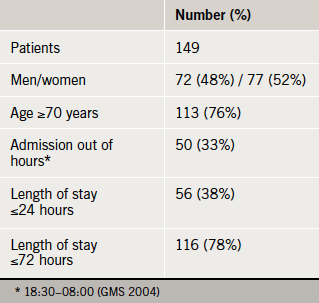
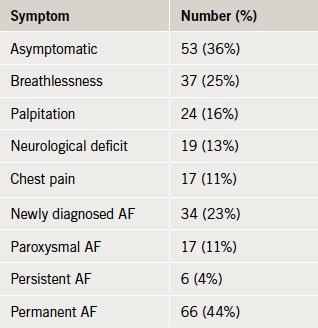
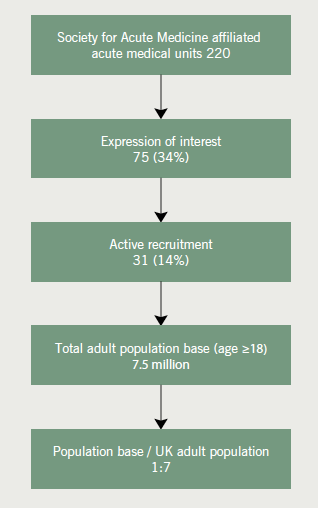
Of the 220 AMUs initially canvassed, 31 eventually provided patient data, representing a population base of more than seven and a half million adult UK residents (figure 1). The electronic questionnaire was completed for 149 patients (table 1). The symptom profile and AF status of the patients is displayed in table 2.
Associated diagnoses
Sepsis was the principal associated diagnosis present in almost a third, followed by heart failure and cerebrovascular events (figure 2). The hierarchy of associated diagnoses was different for the elderly, where cerebrovascular events ranked highest, together with sepsis and heart failure accounting for 70% of presentations (figure 3).
Cardiac investigations
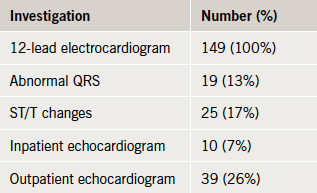
In line with NICE guidelines, all patients received a resting 12-lead electrocardiogram (ECG). Abnormal QRS morphology, predominantly left bundle branch block, was noted in 17%, and repolarisation changes were seen in 13% (table 3).
Stroke risk assessment
Less than a quarter of the study population (23%) had any form of stroke risk assessment (NICE, CHADS2, CHA2DS2-Vasc) (Appendix 2). Retrospective CHA2DS2-Vasc scores for those who were not risk stratified averaged 3.5, and the mean age of this subgroup was 77 years. Of note, 73 (80%) patients with a significant risk for thromboembolic stroke (retrospective CHA2DS2-Vasc score >2) were not prescribed anticoagulation therapy at time of discharge. The risk of stroke needs to be balanced with the risk of major haemorrhage. Despite this, only 36% (n=54) of patients with a CHA2DS2-Vasc score >2, had a HAS-BLED score of ≥3 (8.7 major bleeds per 100 patient-years). In addition, 24% (n=15) of patients at risk of major haemorrhage (HAS-BLED score of ≥3) were discharged on anticoagulation therapy. Four of this group were concomitantly discharged on antiplatelet therapy.
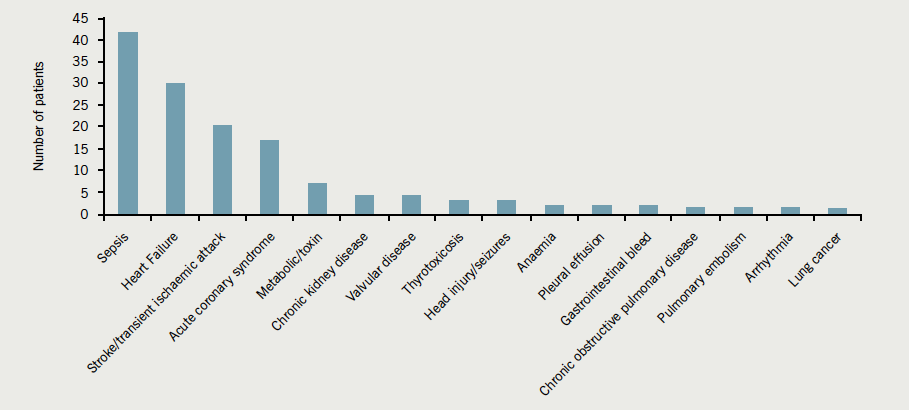
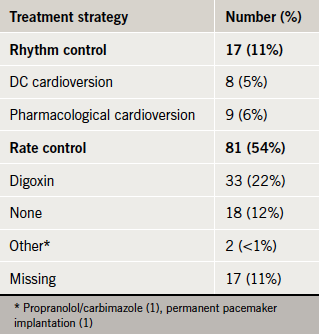
Initial AF treatment strategies
Rate control was the principal form of initial management, but one in five patients received digoxin alone (table 4).
Discharge planning, medication and follow-up
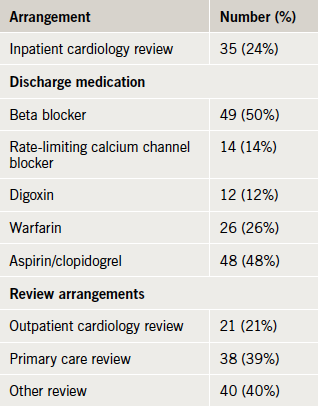
At the time of data collection, information was available for 99 discharged patients (table 5). One in five was offered inpatient or outpatient cardiology review and most were prescribed rate-limiting medication. Half were discharged on aspirin or clopidogrel, and a quarter on warfarin, with the remainder receiving neither an antiplatelet nor an antithrombotic agent.
Out of hours
In total, 49 (33%) of patients presented out of normal working hours (defined as from 6.30 p.m. to 8.00 a.m. on weekdays, the whole of weekends, bank holidays and public holidays).13 Documentation of stroke risk was performed less frequently in patients who presented with AF out of hours, though this was not statistically significant (18.4% vs. 27.2%; Fishers exact p=0.29). Patients who presented with AF out of hours tended not to be discharged within 24 hours (28.6% of patients presenting out of hours vs. 45.7% of patients during normal working hours; Fishers exact p=0.06).
Discussion
The ideal database to study this population would contain the following features:
- Retain the ability for longitudinal study across primary and secondary care
- Continuous data collection direct from clinical records (less sampling bias and case ascertainment issues; reduce duplication/copy error)
- Embedded data validation processes
- Low cost (financial and human resource)
- Ability for rapid feedback to participating organisations
- Reproducible.
There is no existing database that fulfils all these parameters.
Clinical audits and registries have been successfully used to drive quality improvement. Routinely collected data (e.g. hospital episode statistics [HES]) have been used to explore trends in patient outcome. It is estimated that the average cost of running a national clinical audit is £250,000 per year. These audits have reasonably lengthy duration of set up, data collection and analysis, with consequent delay in feedback to participating Trusts. Poor coding practices have also given rise to doubt as to the accuracy of HES data.
We describe the piloting of an electronic survey driven, in part, by the expanding infrastructure of SAM. Our survey was designed to incur low cost and be quick to administer, facilitating rapid data analysis and feedback to participants. Additionally, the methodology allows for ease of replication, allowing time series data to be captured following local improvements. Two thirds of AMUs approached did not express interest in participation, and the eventual contribution rate was low at 14% of the total, but nonetheless, the population base accessed was considerable. We suggest that this model, readily adaptable to different conditions and presentations, has powerful potential as a research tool in acute medical practice.
Our survey suggests that the current management of individuals with AF in the acute medical environment is falling short of national standards7-9 in a number of areas (see Appendix 3).
First, in only a minority did the extended clinical assessment include echocardiography when that information was not already available. Second, a thromboembolic risk assessment was recorded for less than one in four patients. Patients with intermittent or sustained non-valvular AF have similar risks of thromboembolic events.14 Previous studies15 have shown considerable variation in decision-making processes between clinicians with regards to stroke and bleeding risks, as well as choice of antithrombotic therapy, leading to significantly different treatment choices. Findings from RealiseAF, a cross-sectional, international study of >10,000 patients seen over 800 sites, demonstrated that of patients with a CHA2DS2-Vasc score meeting criteria for anticoagulation, the percentage of patients actually receiving anticoagulation were 37.7%, 54.4% and 59.0% for paroxysmal, persistent and permanent AF, respectively.16
Reasons for recalcitrance in antithrombotic therapy in the elderly (who paradoxically are at higher risk of thromboembolic events) stem from safety concerns relating to the risk of falls and major bleeding.17 Several large randomised-controlled trials18,19 have confirmed the efficacy and safety of antithrombotic therapy in this population. The recent systematic review and health economics model of Hughes et al.20 demonstrated that stroke risk stratification models were able to discriminate between different categories of stroke risk (at least 95% confidence interval), and that anticoagulation of high-risk patients was cost-effective. Balancing the risk of stroke in AF with that of major bleeding, Pisters et al.21 have devised a novel bleeding risk score (see Appendix 4), which was validated by Lip et al.22 on a large patient cohort from the Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) III and V trials.
Third, digoxin was the first choice for rate control. Rate control with digoxin is as effective as beta blockers or calcium channel blockers for control of ventricular rate at rest. Beta23 and calcium channel blockers,24 however, have the added advantage of providing effective rate control in exertion (hyperadrenergic state).7,25 In addition, beta blockers have proven efficacy if the patient suffers from concomitant ischaemic heart disease, and diltiazem has been shown to be superior to digoxin in acute AF.26 It is also important to note that while rate control in permanent AF provides alleviation of symptoms, it does not reduce thromboembolic risk.
Fourth, although not expressly stated in the guidelines, but recommended by inference, four out of five patients did not receive a cardiology opinion. EuroHeart, a large scale study (n=5,333) of ambulatory and hospitalised patients with AF seen in cardiology centres across 132 countries demonstrated excellent outcomes.27 Of note, a relatively high proportion of this population (34%) underwent cardioversion at enrolment and 94% had an echocardiogram at least once over the course of a year.
The survey’s demographic findings are in keeping with previous epidemiological data suggesting a higher prevalence of AF in the elderly.28 The typical patient also frequently presents out of hours. Accordingly, the most relevant specialties, cardiology and stroke medicine, are unlikely to be involved in initial, or indeed downstream, management. The primary focus for acute medicine is often an important associated or precipitating diagnosis, such as sepsis. This is in contrast to other national and international studies of AF where chronic comorbidities are more frequently reported, often in ambulatory settings.29
Other management challenges for the acute medical environment include potential hypotensive and negative inotropic effects of beta and calcium channel blockers. ECG monitoring may be standard in acute medical units, but is not commonly accompanied by the nurse to patient ratios that would support aggressive cardiovascular management. In contrast, one study demonstrated that metoprolol was frequently prescribed (in 67.6% of cases) to patients treated for AF in emergency departments within the UK.30
Where acute medicine could undoubtedly improve is in the assessment of thromboembolic risk, particularly with the availability of CHA2DS2-Vasc, a rapid user-friendly scoring system with prognostic power. This said, taking the time to assess risk and translate this to treatment may be challenging in high intensity, rapid turnover units.
Within the different setting of general practice, the percentage of patients prescribed anticoagulants increased from 20 to 34% in men and 17 to 25% in women over four years.31 It is worth noting that general practice databases also hold a large amount of valuable information. This information may be selectively harnessed to aid anticoagulation decision making by using tools such as GRASP-AF. When used alongside data from the Global Anticoagulation Registry in the Field (GARFIELD), an international registry of patients with AF at risk of stroke, GRASP-AF may improve anticoagulation uptake for appropriate patients in the acute medical setting.32
We recognise that this study design has a number of limitations. It is a measurement at a single point of time, with little facility to compensate for variation. It consists of self-reported data, with no ability for external validation. The need to distinguish zero admissions from non-participation became evident at the end of the pilot, as did the need for contextual data (e.g. size of AMU). The dataset is rich with clinical information and process measures, but the design makes clinical outcome measures difficult to measure. Relatively low participation rates limit generalisability of the results.
Despite this, we suggest that this relatively simple study offers valuable insights into the real world management of an important and common condition, and highlights the challenges faced in delivering best evidence into practice, particularly within an acute medical setting •
Acknowledgements
We would like to thank the Society for Acute Medicine for distribution of the electronic survey, all participating units and Dr David Ward, Consultant Physician in Acute Medicine at Queen Elizabeth Hospital, South London Healthcare NHS Trust, for contributing to design of the electronic survey.
Conflict of interest
JS Clinical Research Fellowship is funded by the Royal College of Physicians. AB, DB, DCM: none declared.
Disclaimer
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under the Collaborations for Leadership in Applied Health Research and Care (CLAHRC) programme for North West London. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Key messages
- The first national survey examining the management of atrial fibrillation (AF) within acute medical units in the UK
- Almost a quarter of patients with permanent AF were prescribed digoxin as initial monotherapy. The rationale for this treatment decision was only documented in a third of cases and was not in keeping with national standards
- Stroke risk score was infrequently documented (23%) and retrospective analysis suggests 80% of patients with a significant risk for thromboembolic stroke (CHA2DS2-Vasc score >2) did not receive anticoagulation
- Real world practice differs from national recommendations, further work examining barriers to implementation is crucial
References
- Lip GYH, Tse HF. Management of atrial fibrillation. Lancet 2007;370:604–18. http://dx.doi.org/10.1016/S0140-6736(07)61300-2
- Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.595140
- Heeringa J, van der Kuip DAM, Hofman A et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949. http://dx.doi.org/10.1093/eurheartj/ehi825
- Lip GY, Golding DJ, Nazir M, Beevers DG, Child DL, Fletcher RI. A survey of atrial fibrillation in general practice: the West Birmingham Atrial Fibrillation Project. Br J Gen Pract 1997;47:285–9.
- Stewart S, Hart CL, Hole DJ, McMurray JJV. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–21. http://dx.doi.org/10.1136/heart.86.5.516
- Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. http://dx.doi.org/10.1001/jama.285.18.2370
- National Institute for Health and Clinical Excellence. Atrial fibrillation: the management of atrial fibrillation. CG36. London: NICE, 2006. Available from: http://www.nice.org.uk/CG36
- Fuster V, Rydén LE, Cannom DS et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary. Circulation 2006;114:e257–e354. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.177292
- Camm JA, Kirchhof P, Lip GYH et al. Guidelines for the management of atrial fibrillation. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology. Euro Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
- Loo B, Parnell C, Brook G, Southall E, Mahy I. Atrial fibrillation in a primary care population: how close to NICE guidelines are we? Clin Med 2009;9:219–23.
- Lim JCES, Suri A, Sornalingham S, Chua TP, Lim J. Audit of management of atrial fibrillation at a district general hospital. Br J Cardiol 2010;17:89–92.
- Lambourne A, Percival F, Ward D, Laverty AA, Bell D. An evaluation of consultant input into acute medical admissions management in England. Report of: Hospital service patterns versus clinical outcomes in England. London: Royal College of Physicians, 2012.
- The National Health Service (General Medical Services Contracts) Regulations 2004. 2013.
- Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol 2000;35:183–7. http://dx.doi.org/10.1016/S0735-1097(99)00489-1
- Anderson N, Fuller R, Dudley N. ‘Rules of thumb’ or reflective practice? Understanding senior physicians’ decision-making about anti-thrombotic usage in atrial fibrillation. QJM 2007;100:263–9. http://dx.doi.org/10.1093/qjmed/hcm016
- Chiang CE, Naditch-Brule L, Murin J et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632–9. http://dx.doi.org/10.1161/CIRCEP.112.970749
- Dunn RB. Audit, antithrombotics and atrial fibrillation – going full circle. Age Ageing 2002;31:327–8. http://dx.doi.org/10.1093/ageing/31.5.327
- Mant J, Hobbs F, Fletcher K et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493–503. http://dx.doi.org/10.1016/S0140-6736(07)61233-1
- Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007;36:151–6. http://dx.doi.org/10.1093/ageing/afl129
- Hughes M, Lip G. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. QJM 2007;100:599–607. http://dx.doi.org/10.1093/qjmed/hcm076
- Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest 2010;138:1093–1100. http://dx.doi.org/10.1378/chest.10-0134
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest 2010;137:263–72. http://dx.doi.org/10.1378/chest.09-1584
- DiBianco R, Morganroth J, Freitag JA, Ronan JA, Lindgren KM. Effects of nadolol on the spontaneous and exercise-provoked heart rate of patients with chronic atrial fibrillation receiving stable dosages of digoxin. Am Heart J 1984;108:1121–7. http://dx.doi.org/10.1016/0002-8703(84)90592-1
- Lundstrom T, Rydén L. Ventricular control and exercise performance in chronic atrial fibrillation: effects of diltiazem verapamil. J Am Coll Cardiol 1990;16:86–90. http://dx.doi.org/10.1016/0735-1097(90)90461-W
- Lim HS, Hamaad A, Lip G. Clinical review: clinical management of atrial fibrillation-rate control versus rhythm control. Critical Care 2004;8:271–9. http://dx.doi.org/10.1186/cc2827
- Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med 1997;29:135–40. http://dx.doi.org/10.1016/S0196-0644(97)70319-6
- Pisters R, Nieuwlaat R, Prins MH et al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace 2012;14:666–74. http://dx.doi.org/10.1093/europace/eur406
- Hobbs FDR, Fitzmaurice D, Mant J et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9:iii–iv, ix–x, 1–74.
- Freestone B, Rajaratnam R, Hussain N, Lip GYH. Admissions with atrial fibrillation in a multiracial population in Kuala Lumpur, Malaysia. Int J Cardiol 2003;91:233–8. http://dx.doi.org/10.1016/S0167-5273(03)00031-7
- Rogenstein C, Kelly AM, Mason S et al. An international view of how recent-onset atrial fibrillation is treated in the emergency department. Acad Emerg Med 2012;19:1255–60. http://dx.doi.org/10.1111/acem.12016
- Majeed A, Moser K, Carroll K et al. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart 2001;86:284–8. http://dx.doi.org/10.1136/heart.86.3.284
- Kakkar AK, Mueller I, Bassand JP et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13.e1–9.e1. http://dx.doi.org/10.1016/j.ahj.2011.09.011