This year’s European Society of Cardiology (ESC) Congress promised the strongest scientific programme yet and it did not disappoint. With 4,598 posters and oral presentations, much excitement surrounded the reporting of the 27 studies in the five hot line sessions. While results from PARADIGM and ODYSSEY look set to be major breakthroughs in the treatment of heart failure and cholesterol lowering, respectively, the results from SIGNIFY caused much debate over the role of heart rate in cardiovascular disease. We report some of the highlights of this year’s Congress attended by over 30,000 delegates and held in Barcelona, Spain, from 30th August to 2nd September 2014. Our podcasts (available here) put some of the key studies in perspective for UK practice.
A PARADIGM shift in heart failure therapy
 A new drug representing a novel therapeutic concept has shown extremely good results in a phase 3 trial in heart failure, generating much excitement.
A new drug representing a novel therapeutic concept has shown extremely good results in a phase 3 trial in heart failure, generating much excitement.
The drug, referred to as an angiotensin receptor-neprilysin inhibitor (ARNI), combines the neprilysin inhibitor sacubitril (AHU377) and the angiotensin receptor blocker (ARB), valsartan. Neprilysin is a neutral endopeptidase involved in the metabolism of a number of vasoactive peptides. Developed by Novartis and known only as LCZ696 at present, the new agent could be on the market next year.
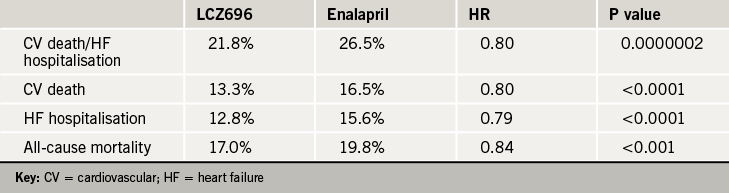
In the current study known as PARADIGM-HF, LCZ696 showed significant and sizable reductions in death from cardiovascular causes and hospitalisation for heart failure compared with the ACE-inhibitor, enalapril.
Co-primary investigator, Dr John McMurray (University of Glasgow, UK) said: “This really is an astonishing result and a real breakthrough for patients with heart failure.”
The other co-primary investigator, Dr Milton Packer (University of Texas Southwestern Medical Center, Dallas, USA) commented: “To say that we are excited is an understatement. We are absolutely thrilled.” He added: “Given the survival advantage of LCZ696 over currently available drugs, once this drug becomes available, it would be difficult to understand why physicians would continue to use traditional ACE inhibitors or ARBs for the treatment of heart failure.”
The PARADIGM trial randomised 8,399 patients with class II to IV heart failure and an ejection fraction of 40% or less to either LCZ696 200 mg twice daily or enalapril 10 mg twice daily in addition to recommended therapy.
The trial was stopped early after a median follow-up of 27 months by the data safety monitoring committee because of “overwhelming benefit” in the LCZ696 group. Full details of the results show that the primary end point – death from cardiovascular causes or hospitalisation for heart failure – was reduced by 20% with LCZ696 (see table 1).
With regard to adverse effects, there was more symptomatic hypotension in the LCZ696 group (14% vs. 9.2%) but this rarely required the discontinuation of treatment. In fact, fewer patients in the LCZ696 group stopped their study medication for any adverse event (10.7% vs. 12.3%). LCZ696 was not associated with an increased risk of serious angioedema, which was the main safety concern observed with a related medication – omapatrilat – in the OVERTURE trial.
Dr Packer explained that omapatrilat differed from LCZ696 in that omapatrilat inhibited ACE, neprilysin and aminopeptidase P, and that LCZ696 was specifically designed to minimise the risk of serious angioedema by combining ARB and neprilysin inhibition.
Approval applications for the new agent are due to be completed in the US by the year end, and should be filed in Europe early next year.
The PARDIGM-HF trial was published in the September 11th issue of the New England Journal of Medicine. A perspective accompanying the publication says the PARADIGM-HF trial may represent “a new era of treatment for heart failure.”
SIGNIFY questions role of ivabradine in angina
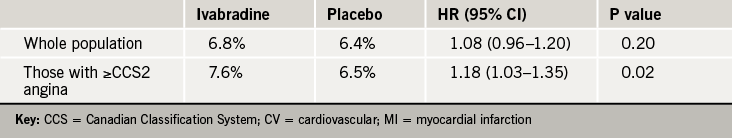
Reducing the heart rate with ivabradine had no effect on reducing cardiovascular events when added to guideline-based treatment in patients with stable coronary artery disease without clinical heart failure in the SIGNIFY trial.
In fact, in patients with more severe angina (CCS class ≥II), which made up more than half the study population, ivabradine was associated with a worse outcome, a result which caused much surprise and confusion at the ESC meeting, given that it is approved for use in the treatment of angina in many countries worldwide.
During a panel discussion after the presentation of the trial, several experts said the results led to questions about a fundamental belief in cardiology – that lowering heart rate was generally beneficial.
Lead author of the trial, Dr Kim Fox (Royal Brompton Hospital, London) said the explanation for this “surprising finding” in the severe angina group is uncertain, but that it should be treated with caution since the results of the primary efficacy analysis were not significant
He suggested that whether lowering heart rate was beneficial or not probably depended on the individual population. He pointed out that ivabradine had previously shown improved outcomes in patients with heart failure, and it also improves angina symptoms, but the SIGNIFY results show that “lowering the heart rate in patients with stable coronary disease without heart failure does not prevent the progression of coronary artery disease.”
In the publication of the study in the New England Journal of Medicine (online on August 31st), the SIGNIFY authors conclude that an elevated heart rate may be only a marker of risk rather than a modifiable determinant of outcomes in patients who have stable coronary artery disease without clinical heart failure.
The SIGNIFY trial randomised 19,102 patients with a heart rate of 70 beats per minute or more to ivabradine or placebo. The dose of ivabradine was adjusted to achieve a target heart rate of 55 to 60 beats per minute up to a maximum of 10 mg twice daily.
After a median follow-up of 27.8 months, results showed no significant difference in the primary end point – cardiovascular death or non-fatal myocardial infarction – between the ivabradine group and the placebo group (6.8% vs. 6.4%, HR=1.08, p=0.20). Within the population of 12,000 patients with more severe angina, the ivabradine group showed a significant increase in the primary outcome (table 1).
One of the panel discussants of the trial at the Barcelona meeting, Dr Thomas Lüscher (University Hospital Zurich, Switzerland), suggested that ivabradine may have lowered heart rate too much in some patients. Noting that a very slow heart rate may endanger coronary flow, he called for an analysis of the distribution of heart-rate reduction to see if to see if the lowest rates had the most events.
Ivabradine is currently undergoing a “re-assessment” at the European Medicines Agency, which says it is reviewing how the data from the SIGNIFY study “impact the balance of benefits and risks of ivabradine”. The Agency notes that the dose used in SIGNIFY was higher than the approved dose and reminds that the starting dose of ivabradine is 5 mg twice daily, and the maintenance dose should not exceed 7.5 mg twice daily. It recommends doctors monitor patients carefully for bradycardia and reduce the dose if heart rate falls persistently below 50 beats per minute.
In an editorial accompanying publication of the study in the New England Journal of Medicine, Drs Magnus Ohman and Karen Alexander (Duke University, Durham, USA) note that ivabradine did significantly reduce the occurrence of angina in the severe angina cohort, but that because of the outcome results, doctors should exercise caution among patients with more severe forms of angina and to consider adjusting beta blocker doses to effective levels before initiating ivabradine.
They point out that in the heart failure trial, the majority of benefit with ivabradine was among patients who could not take beta blockers or who were taking a lower dose. “Whether this holds true for patients with angina is unknown, but this cautious approach may be reasonable until we understand this finding better,” they suggest.
ODYSSEY: PCSK9 inhibitors herald new era in cholesterol reduction

PCSK9 (proprotein convertase subtilisin kexin 9) was a hot topic in Barcelona, reflected by the inclusion of four trials from the ODYSSEY phase III programme with alirocumab, a fully human monoclonal antibody to PCSK9, in the hotline sessions.
All four trials were in patients at high to very high risk of cardiovascular disease, whose clinical needs were unmet with conventional lipid-lowering approaches: ODYSSEY FH I and FH II (in familial hypercholesterolaemia [FH]), ODYSSEY COMBO II (in high-risk patients with non-familial hypercholesterolaemia), and ODYSSEY LONG TERM (in both FH and non-FH patients).
Overall, these trials showed substantial sustained low-density lipoprotein (LDL) cholesterol lowering, together with no adverse signal, results which were hailed as “remarkable” and “impressive” by discussant Professor Thomas Luscher (University Hospital, Zurich, Switzerland). Key results from these important studies are summarised in table 1.

Online extra

These studies are also the focus of a special report online, which rounds up all the lipids news from the congress. In our ESC podcast, we interview one of the ODYSSEY investigators, Dr Christopher P Cannon (Harvard Clinical Research Institute, Boston, USA), who puts these studies into clinical perspective, and also speak to Professor Kausik Ray (Division of Cardiovascular Sciences, St. Georges Hospital, University of London), who explains why the extent of LDL cholesterol lowering is key in the prevention of cardiovascular disease, rather than the means of LDL cholesterol lowering.
CONFIRM: treat iron deficiency to improve heart failure
Many heart failure patients can achieve improvements in functional capacity, symptoms and quality of life, simply by treatment with iron supplements, according to a new randomised trial.
Presenting the CONFIRM study, Dr Piotr Ponikowski (Medical University Wroclaw, Poland) noted that iron deficiency, which affects around half of heart patients, has been associated with impaired functional capacity, poor quality of life, and increased mortality, irrespective of the presence of anaemia. “Correction of iron deficiency is therefore an attractive therapeutic target,” he said.
In the study, 304 patients with New York Heart Association (NYHA) class 2–3 heart failure and serum ferritin <100 ng/mL, or 100–300 ng/mL if transferrin saturation was <20%, were randomised to ferric carboxymaltose as intravenous injections of 10 or 20 mls (equivalent to 500 or 1,000 mg of iron, respectively) or placebo. Study drug was given in doses based on subject weight and haemoglogin value at screening. Total doses were between 500 and 2,000 mg of iron in the therapy phase (dosed at baseline and week 6), and thereafter maintenance dosing of 500 mg iron at each of weeks 12, 24, and 36, if iron deficiency was still present.
In the treatment arm, the median total dose was 1,500 mg of iron during the one-year study period. Over 75% of the patients required a maximum of two injections to correct and maintain the iron parameters.
Treatment with iron was associated with a significant improvement in six-minute walking test distance at week 24 (the primary end point) by 33 metres versus placebo. The treatment group achieved an increase of 18 metres walking distance compared with a 16 metre reduction in the placebo group. This effect was consistent in all subgroups and was sustained to week 52.
Patients treated with iron also showed an improvement in NYHA class, patient global assessment, quality of life and fatigue score throughout the study. There was also a significant reduction in the risk of hospitalisations for worsening heart failure (hazard ratio 0.39) . The iron treatment was well tolerated with no difference in adverse effects between the active and placebo groups.

Dr Ponikowski noted that current ESC guidelines for managing heart failure patients recognise iron deficiency as a common and clinically relevant comorbidity, which should always be tested for. However at the same time, there is a relatively weak recommendation to manage iron deficiency in such patients, mainly because of a paucity of evidence confirming the benefits of iron repletion. “CONFIRM-HF was designed to provide that evidence, and we believe that the results of this trial will be most important when it comes to defining the role of iron repletion for heart failure iron deficient patients in the next ESC Heart Failure guidelines in 2016,” he said.
The CONFIRM study was published online in the European Heart Journal on August 31st. Professor Keith Fox, Chair of the ESC Programme Committee, discusses the CONFIRM study in one of our ESC podcasts.
X-VERT: rivaroxaban an alternative to VKA in cardioversion for AF
Oral anticoagulant therapy with rivaroxaban is a safe alternative to vitamin K antagonists (VKAs) in patients with atrial fibrillation (AF) who are undergoing elective cardioversion according to the results of the X-VERT study. In addition, rivaroxaban may potentially have one important advantage over VKAs, with a shorter time to cardioversion, the study suggests.
Currently many patients with AF are not able to undergo cardioversion since treatment with VKAs – the current standard of care – requires at least three weeks of treatment to achieve adequate anticoagulation.
Professor Riccardo Cappato (University of Milan, Italy), the co-principal investigator who presented the results, said X-VERT had shown that time to cardioversion was shorter with rivaroxaban compared to VKA in both study arms, with it being significantly shorter in the delayed strategy arm of the study.
X-VERT is the first prospective, randomised trial to compare the safety and efficacy of rivaroxaban compared to VKA therapy in cardioversion.
It was hailed as a “landmark trial in the development of NOACs” by discussant Professor Christoph Bode (Univeristy of Freiburg, Germany) who said there had previously only been post-hoc data for the use of NOACs and VKAs in cardioversion.
Carried out in 141 centres and 16 countries on patients with AF scheduled to undergo either electrical (97.6%) or pharmacological (2.4%) cardioversion, X-VERT randomised 1,002 patients to oral rivaroxaban 20 mg once daily and 502 patients to VKA (warfarin or another VKA at the investigator’s discretion, based on local standard of care). Using established guidelines, patients were assigned to either early (one to five days) or delayed (21 to 25 days) cardioversion after randomisation.
The study found that, patients treated with rivaroxaban had a similar of risk of the primary composite outcome (stroke or transient ischaemic attack, peripheral embolism, myocardial infarction, and cardiovascular death) to patients taking a VKA (0.5% versus 1.02%, respectively), which was not statistically significant.
There was no difference in major bleeding between the rivaroxaban and VKA groups (0.61% versus 0.80%, respectively; RR 0.76), and also no clinically important differences of adverse events by treatment assignment or by cardioversion strategy.
The study did, however, show that time to cardioversion was significantly shorter with the delayed strategy when patients were treated with rivaroxaban compared to the VKA-treated patients (median 22 versus 30 days, respectively, p < 0.001).

FOCUS-2: polypill increases adherence to post-MI treatment
Taking the multiple medications needed after a myocardial infarction (MI) as one ‘polypill’ increased adherence to secondary prevention therapy and therefore could lead to a reduced rate of recurrent events, the results of the FOCUS-2 study showed.
Presenting the data, Dr Valentin Fuster (Mount Sinai Heart, New York, US) noted that improved secondary prevention treatments after MI has contributed significantly to the reduced cardiovascular mortality rate seen over the pervious 20 years in western countries, but that lack of adherence to treatment impedes adequate secondary prevention and contributes to the cardiovascular pandemic.
He said: “The most important factors responsible for a lack of adherence are the complexity of treatment and the daily number of prescribed pills. A polypill could simplify healthcare delivery, improve cost-effectiveness, support the comprehensive prescription of evidence-based cardioprotective drugs, and reach underdeveloped regions of the world.”
In the FOCUS-2 study, 695 patients were randomised to receive either a polypill containing aspirin 100 mg, simvastatin 40 mg and ramipril 2.5, 5 or 10 mg, or the three medications separately.
After nine-months follow up, those who were taking the polypill were found to have higher adherence rates. Self-reported questionnaires showed that 68% of patients in the polypill group took their medication compared to 59% of patients taking the drugs separately.
The researchers also counted pills left, and this method found that 92% of patients in the polypill group were adherent compared to only 84% in the group assigned to separate drugs.
The study is continuing and will assess whether there are any differences between the two treatment arms in blood pressure, cholesterol, safety or costs.
Dr Fuster concluded: “Our results suggest that the polypill has the potential to prevent more patients having a second heart attack. A randomised trial is needed to test whether the improved adherence with the polypill found in FOCUS results in fewer post MI patients having another MI.”
ATLANTIC – prehospital ticagrelor for STEMI?

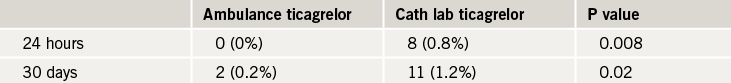
Giving the antiplatelet agent, ticagrelor, to ST-elevation myocardial infarction (STEMI) patients in the ambulance was safe but did not improve pre-percutaneous coronary intervention (PCI) coronary reperfusion compared to giving it in the cath lab, results of the ATLANTIC trial show.
However, there was a reduction in post-PCI stent thrombosis in the ambulance group.
The study, was published online in the New England Journal of Medicine on September 1st to coincide with its presentation in Barcelona.
The authors, led by Dr Gilles Montalescot (Pitie-Salpetriere Hospital, Paris, France), point out that all the stent-thrombosis events within the first 24 hours occurred in the in-hospital group, and the difference remained significant in favour of pre-hospital administration of ticagrelor for up to 30 days. They note that the maximal difference in platelet inhibition occurred concomitantly with the reduction of stent thrombosis.
“The rate of definite stent thrombosis was reduced without a safety trade-off,” they state. They caution that prespecified, stent thrombosis was a secondary end point among neutral study results; therefore, this finding should not be interpreted as definitive.
The ATLANTIC study included 1,862 STEMI patients within six hours of symptom onset who were randomised to ambulance or in-hospital treatment with ticagrelor. The ambulance loading dose was 180 mg ticagrelor before transfer, then a matching placebo in the cath lab. The other group received placebo in the ambulance then a 180 mg ticagrelor loading dose in hospital. All patients subsequently received 90 mg ticagrelor twice daily for 30 days. The median time difference between the two treatment strategies was 31 minutes.
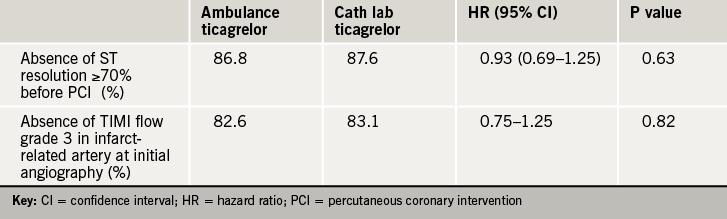
The study findings showed no difference between the groups for the co-primary end points – the proportion of patients who did not have a 70% or greater resolution of ST-segment elevation before PCI and the proportion of patients who did not have a TIMI flow of grade 3 in the infarct-related artery at initial angiography (tables 1 and 2).
Home monitoring ICDs – benefits without costs increase
Remote (home) monitoring of pacemakers and implantable cardiac defibrillators (ICDs) is increasingly popular in the UK and other countries. Now the first financial assessment of the impact of home monitored follow-up suggests that the cost to physicians, hospitals and insurance providers is the same as traditional in-office monitoring, according to a new study conducted by Dr Hein Heidbuchel, (Heart Center Hasselt, Belgium) and co-workers.
EuroEco (The European Health Economic Trial on Home Monitoring in ICD Therapy) identified wide variations in financial costs in switching to this approach, due to national differences in insurance reimbursement.
Reimbursement, which occurs in Germany and the UK, seems to be a key determinant in adopting this technology and the main objective of the study was to evaluate the costs of home monitoring (HM)-based follow-up, using a Biotronik home monitoring system, compared to in-office follow-up.
The study had a randomised, non-blinded, parallel-design, involving 17 centres from Belgium, Germany, Great Britain, Spain, and The Netherlands, and enrolled 303 patients (average age 62.4 years, 81% male) who were scheduled to receive a single- or dual-chamber ICD equipped with home monitoring (HM) technology.
After ICD placement, patients were randomised to either a HM ON group (n=159), where there was continuous automatic remote monitoring. or a HM OFF group (n=144), where HM was in place but switched off. They were followed for two years. Despite a significantly higher number of office visits that were unscheduled in the HM ON group, the total number of visits was still significantly lower for HM ON compared to HM OFF patients (3.79 vs. 5.53, p<0.001). Patient self-reported quality of life was not different between the two groups.
The total follow-up cost for providers (the primary endpoint of the trial) was not different for HM ON versus HM OFF (mean €204 vs. €213, respectively), neither was their net profit (€408 vs. €400). The costs for the remote monitoring equipment and communication, now picked up by industry were not included in the calculations.
As the study showed that Home Monitoring reduces hospitalisations and length of stay, Dr Heidbuchel expressed hope that this may lead to “balanced reimbursement scenarios in order to stimulate reorganisation towards remote monitoring-based care”.
And now, a new battery-free cardiac pacemaker!
A new ‘batteryless cardiac pacemaker’ based on an automatic wristwatch and powered by heart motion was presented at the congress. The prototype device does not require battery replacement.
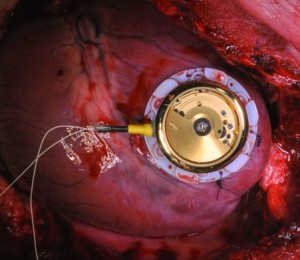
“Batteries are a limiting factor in today’s medical implants. Once they reach a critically low energy level, physicians see themselves forced to replace a correctly functioning medical device in a surgical intervention. This is an unpleasant scenario which increases costs and the risk of complications for patients,” Mr Adrian Zurbuchen (University of Berne, Switzerland) said at the congress.
Using heart motion as an alternative energy source, the first prototype is based on a commercially available automatic wristwatch, with a custom-made housing containing eyelets to allow suture of the device directly onto the myocardium (pictured).
The prototype works the same way as it would on a person’s wrist. When it is exposed to an external acceleration, the eccentric mass of the clockwork starts rotating. This rotation progressively winds a mechanical spring. After the spring is fully charged, it unwinds and thereby spins an electrical micro-generator. Energy is temporarily stored in the buffer capacity; the buffered energy is used by the pacemaker to apply minute stimuli to the heart.
The researchers successfully tested the system in in vivo experiments with domestic pigs. The newly developed system allowed them for the first time to perform batteryless overdrive-pacing at 130 beats per minute.
Mr Zurbuchen said: “We have shown that it is possible to pace the heart using the power of its own motion. The next step in our prototype is to integrate both the electronic circuit for energy storage and the custom-made pacemaker directly into the harvesting device. This will eliminate the need for leads.”
Obese children have six-fold increased risk of hypertension
Obese children and adolescents have up to a six-fold greater risk of hypertension than those of normal weight, with the effect greatest in girls, a new study has shown.
Presenting the data, Professor Peter Schwandt (Ludwig-Maximilian University of Munich-Nuremberg, Germany), said: ‘When using per cent body fat to define obesity we found that obese girls had a nearly six-fold higher risk of hypertension than normal weight girls. In obese boys the risk was more than four times greater than their normal weight counterparts.”
The study included 22,051 children and adolescents from the Prevention Education Program Family Heart Study, a prospective community-base observational study conducted in Nuremberg, Germany. The researchers measured blood pressure, body mass index (BMI), waist circumference, waist-to-height ratio, skinfold thickness, and per cent body fat.
Hypertension was defined as a blood pressure reading over the 95th percentile of the blood pressure curve for children and adolescents. Prehypertension was defined as blood pressure between the 90th and 95th percentile.
When using elevated BMI to define overweight and obesity, the researchers found that the risk of prehypertension was increased by 1.6 in those who were overweight; by 2.4 in obese boys, and by 3.3 in obese girls.
This increased risk was similarly found with other measures of body fat – elevated skinfold thickness, weight-to-height ratio, and abdominal adiposity.
Consider gender and age in resistant hypertension patients
Gender and age should be added to the risk stratification of resistant hypertension, a Taiwanese study suggests.
In the study, researchers analysed data from 120,000 subjects in a national health database. They found those with resistant hypertension had a 17% greater risk of major adverse cardiovascular events than those with non-resistant hypertension.
Subgroup analysis showed that resistant hypertension increased the risk of stroke in females by 35% and in elderly patients by 20% – though not in young or male patients.
Investigator Dr Kuo-Yang Wang (Taichung Veterans General Hospital, Taichung, Taiwan) said this was the first study to explore the relationship between gender, age, and cardiovascular events in patients with resistant hypertension and that “diagnosis of resistant hypertension should be combined with patient gender and age to provide a more accurate prediction of stroke risk.”
More reports from ESC
Major trials and their relevance for practice are covered in our ESC podcasts:
- Professor Kausik Ray (St George’s Hospital, University of London) and Dr Christopher Cannon (Harvard Clinical Research Institute, Boston, USA) give further insights into lipid-lowering and the ODYSSEY studies.
- Professor Keith Fox (University of Edinburgh, and Chairman of the ESC Programme Committee) talks to Dr Afzal Sohaib (Imperial College London, and President of the British Junior Cardiologists’ Association) on: PARADIGM-HF and CONFIRM-HF, in heart failure; SIGNIFY, ATLANTIC, CULPRIT, MITOCARE, COMPLETE and TASTE in coronary artery disease; X-VERT and STICS in arrhythmia; and ODYSSEY and SOLID-TIMI 52 in lipid lowering