Identification of those at low risk of developing heart failure (HF) after acute coronary syndrome (ACS) would aid clinical management, but it is unclear whether N-terminal pro-brain natriuretic peptide (NT-proBNP) adds to the predictive accuracy of troponin. There were 229 subjects recruited into a prospective cohort study. Subjects were assessed for acute heart failure (AHF) prior to discharge and for readmission within 30 days of their ACS event (cohorts A+B). Cohort A (n=116) were further assessed for readmission within 12 months. Troponin I (TnI) and NT-proBNP levels were measured at ACS onset and at 6–12 hours. Readmissions were identified using electronic records. In total, 23.6% of subjects developed AHF during the index admission: 10.0% were readmitted within 30 days of admission; 17.2% within three months; 26.7% within six months and 36.2% within 12 months. At presentation, NT-proBNP, but not TnI, was significantly elevated among subjects who developed AHF compared with non-AHF subjects. Compared with non-readmitted subjects, readmission within 30 days was associated with significantly lower baseline NT-proBNP, and readmission after 30 days with higher baseline NT-proBNP. For all periods, TnI level was lower among readmitted compared with non-readmitted subjects. In conclusion, NT-proBNP has a potential role for rule out of those at low risk of AHF development and readmission.
Introduction
 For patients with acute coronary syndrome (ACS) who survive to reach hospital, the majority of mortality and morbidity over the following five years occurs after discharge.1 Of all complications, development of acute heart failure (AHF) and left ventricular systolic dysfunction (LVSD) are key determinants of adverse outcome. Approximately half of patients with ACS are readmitted to hospital, constituting a profound burden on healthcare resources.1 In several healthcare systems there are financial penalties when ACS patients are readmitted within 30 days.2 Prediction of the development of AHF and hospital readmission following ACS would aid clinical management, but widely used biomarkers have limited accuracy. Troponin is the only biomarker routinely measured in ACS management despite evidence that more reliable predictors of ACS complications exist.3-6
For patients with acute coronary syndrome (ACS) who survive to reach hospital, the majority of mortality and morbidity over the following five years occurs after discharge.1 Of all complications, development of acute heart failure (AHF) and left ventricular systolic dysfunction (LVSD) are key determinants of adverse outcome. Approximately half of patients with ACS are readmitted to hospital, constituting a profound burden on healthcare resources.1 In several healthcare systems there are financial penalties when ACS patients are readmitted within 30 days.2 Prediction of the development of AHF and hospital readmission following ACS would aid clinical management, but widely used biomarkers have limited accuracy. Troponin is the only biomarker routinely measured in ACS management despite evidence that more reliable predictors of ACS complications exist.3-6
Brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) have been proposed as alternatives to troponin for predicting heart failure (HF) in ACS patients.7-10 They are produced by cleavage of their common precursor, proBNP, after its release from cardiac ventricles.11 Measurement of NT-proBNP within the first few days after ACS provides valuable prognostic information, independent of left ventricular ejection fraction. De Lemos et al.9 demonstrated that baseline BNP level was higher among subjects who experienced new or worsening HF within 30 days or 10 months of their ACS event compared with subjects who did not. Of note, few studies have evaluated the predictive value of baseline NT-proBNP level in early HF, specifically that developing during index admission.
Study rationale
To determine whether biomarkers (troponin I [TnI] and NT-proBNP) are useful in identifying risk of developing AHF and hospital readmission following ACS.
Hypotheses
NT-proBNP is superior to TnI for predicting development of AHF and hospital readmission after ACS.
Surrogate markers of LVSD, e.g. developing AHF during index admission, pre-existing ischaemic heart disease (IHD), predispose to hospital readmission.
Materials and methods
Subjects
The study population comprised
229 subjects. Patients presenting to the Royal Infirmary of Edinburgh with possible ACS were consented and prospectively recruited in two phases during November to December 2011 (cohort A, n=116), and February to March 2013 (cohort B, n=113). The use of two cohorts is a product of the compilation of two student-led projects, and takes advantage of examining the outcomes in two independent, temporally separated patient cohorts.
Patients who received a non-ACS diagnosis at discharge were excluded. Patients with a pre-existing diagnosis of HF were excluded so that any clinical evidence of AHF could be attributed to the concurrent ACS.
Data collection
Data, including clinical history and examination, cardiovascular risk factors, comorbidities and medications, were collected from subjects’ medical notes.
Subjects were classified into three groups according to admission electrocardiogram (ECG) and serial TnI values: unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), consistent with European Society of Cardiology (ESC) guidelines.12,13
Subjects were assessed for evidence of new-onset AHF using clinical examination findings, chest radiography (CXR) and echocardiography. Significant signs of AHF for each modality are bulleted in table 1, which was developed from ESC AHF diagnosis guidelines.14 Presence of at least one significant sign in at least two assessment modalities constituted a diagnosis; symptoms were not sufficient.

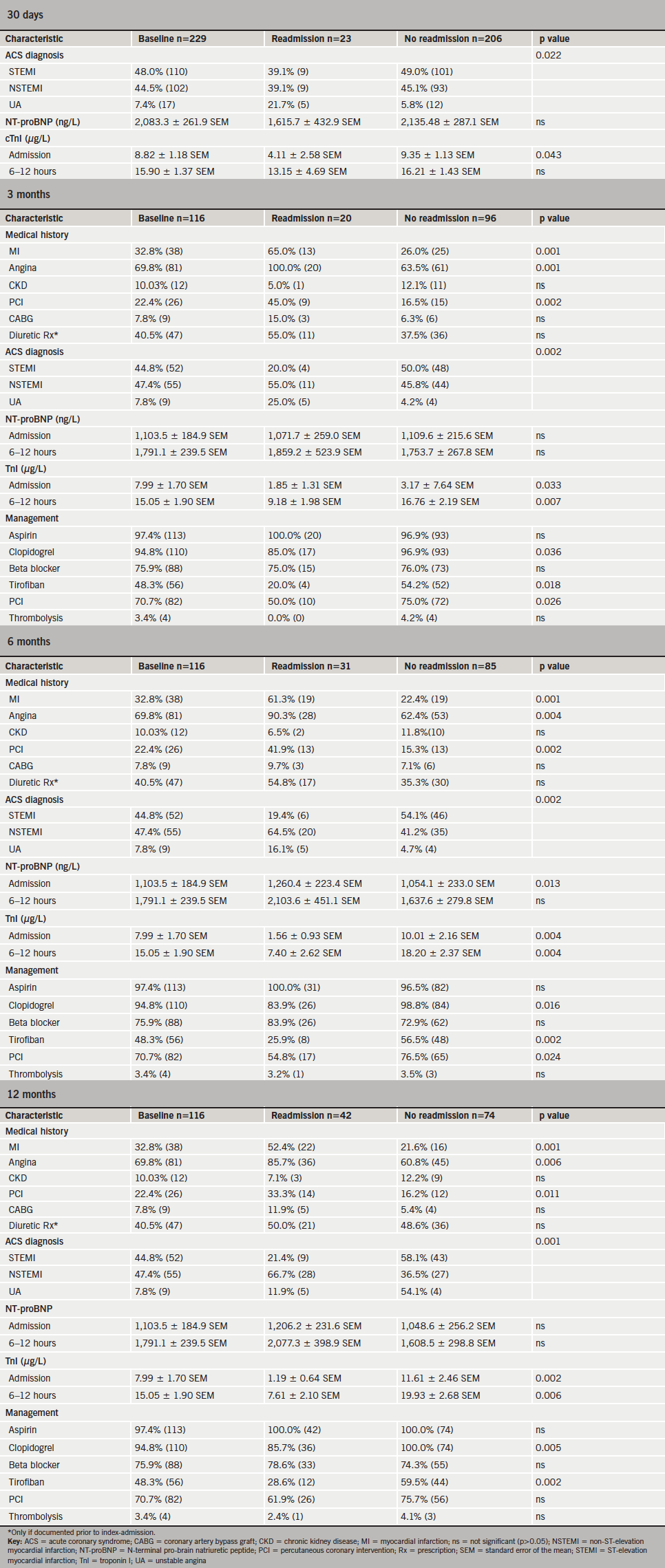
Medical records were accessed post-discharge to identify readmission to hospital within 30 days (cohorts A and B) and within three, six and 12 months (cohort A only) of the ACS event. A readmission is defined as an episode of any cause of elective or emergency admission to hospital.
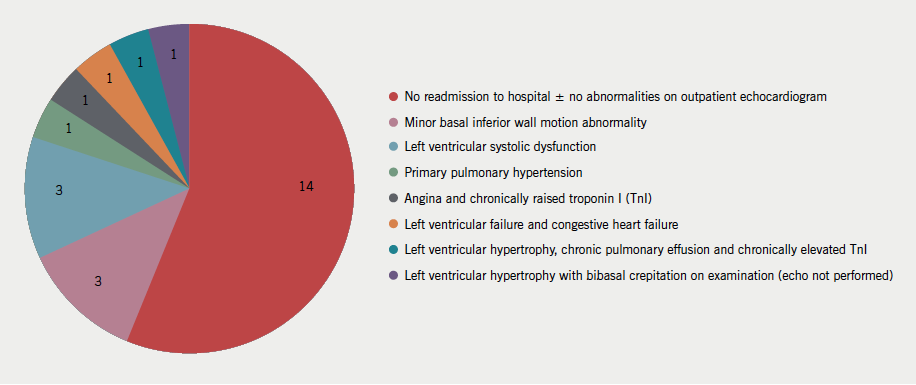
Medical records of the 25 subjects in cohort A who did not develop AHF during index admission despite an admission NT-proBNP level of >2 × 97.5th percentile were accessed at 22 months to determine subsequent AHF development.
NT-proBNP assay
In current practice, venous blood samples are taken on admission for immediate analysis of TnI in patients presenting with possible ACS using the ARCHITECT® STAT chemiluminescent microparticle immunoassay. For non-STEMI patients a further sample is collected at 6–12 hours to reassess TnI level. Aliquots were collected from these samples and assayed for NT-proBNP levels using the Elecsys proBNP II assay. Levels were considered against evidence-based age- and sex-specific reference ranges.
Investigators and treatment-providing clinicians were blinded to NT-proBNP results at the time of assessment.
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics 19. The Chi-squared test of association determined significance for categorical data where >80% of expected counts were >5. Otherwise, Fisher’s exact test was used. The Shapiro-Wilk test generated histograms to determine continuous variable normalities. The two-tailed t-test compared those showing parametric distribution. The biomarkers largely showed non-parametric distribution so were analysed using the Mann-Whitney U test.
Results
The study population comprised 229 subjects. Baseline characteristics of cohorts A and B did not differ significantly, except that cohort A had a higher prevalence of IHD (data not shown).
Development of AHF during index admission for ACS
This study’s criteria for a diagnosis of AHF during index admission were met by 23.6% of subjects (n=54). Cohort A, where 20.7% (n=24) developed AHF, was considered in greater detail. The baseline characteristics of Cohort A are summarised in table 2.
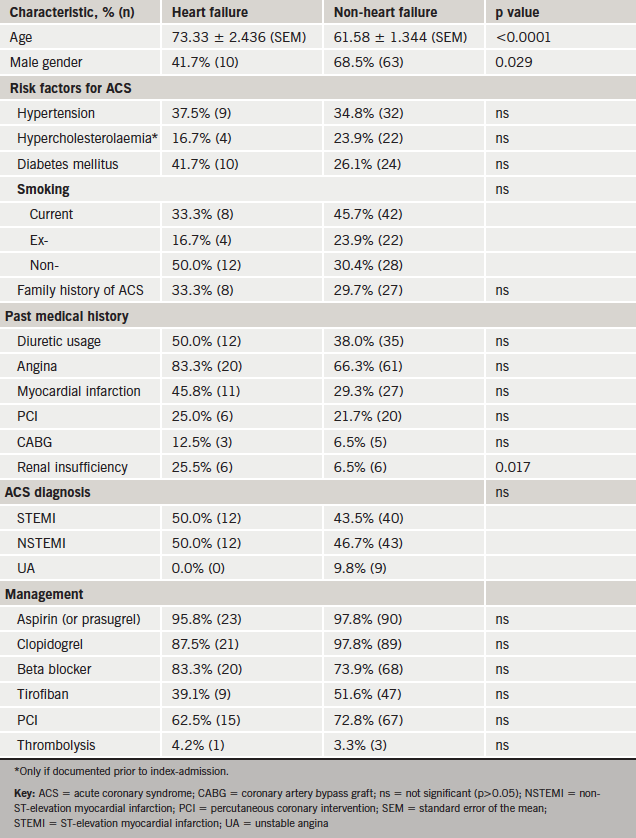
Subjects with HF were significantly older than non-HF subjects, less likely to be male and more likely to have pre-existing renal insufficiency.
NT-proBNP level on admission was significantly higher among subjects who developed AHF during index admission compared with subjects who did not (p<0.0001) (figure 1). This was also true of NT-proBNP levels 6–12 hours after symptom onset (data not shown). By contrast, TnI was not significantly higher in the AHF group on admission (p=0.081) (figure 2) or at 6–12 hours (data not shown) (figure 2).



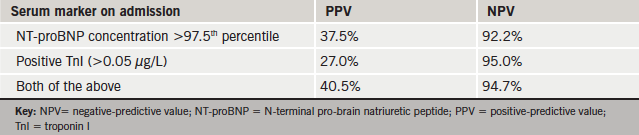
Venn diagrams were compiled for admission TnI and NT-proBNP levels in the HF and non-HF groups (figure 3). Data were analysed to calculate the positive-predictive value (PPV) and negative-predictive value (NPV) of serum markers for predicting HF (table 3). The NPVs of TnI and NT-proBNP exceeded 90% when used individually or combined.
By contrast, the PPVs for both markers were low (table 3). Twenty-five non-AHF subjects had an admission NT-proBNP concentration exceeding twice the upper reference range limit (figure 3). The clinical outcome of these 25 patients at 22 months is represented in figure 4.
Readmission to hospital within 12 months
The data from both cohorts for 30-day readmission rates are considered together. Altogether 10.0% (n=23) of subjects were readmitted within 30 days of the index ACS event. Among cohort A, 17.2% (n=20) were readmitted within three months, 26.7% (n=31) within six months and 36.2% (n=42) within 12 months of ACS. The majority of these were emergency readmissions, predominantly to medical firms (general medicine or cardiology). Complaints included recurrent chest pain or shortness of breath. These are detailed further in appendix 1.
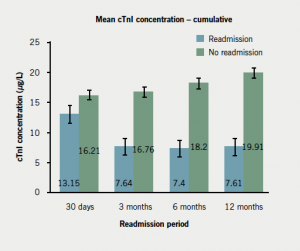
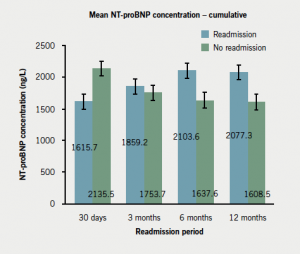
Compared with non-readmitted subjects, readmissions within 30 days had lower TnI levels on index admission (p=0.043); lower mean NT-proBNP levels (not significant) (figure 5; appendix 3); and were more likely to be diagnosed with UA (appendix 3). Readmissions beyond 30 days were more likely to have a history of IHD (particularly angina); significantly lower TnI level at baseline (figure 5; appendix 3); and were less likely to have been diagnosed with STEMI compared with non-readmissions (appendix 3). In keeping with this, those readmitted after three months were less likely to have received tirofiban (reserved for STEMI patients). Those readmitted within three to six months were less likely to have undergone percutaneous coronary intervention (PCI). Readmissions after 30 days had significantly higher baseline NT-proBNP level compared with non-readmissions (figure 6; appendix 3).
The NPV of NT-proBNP for readmission within 30 days exceeded 90%, though its value subsequently decreased (table 4). By contrast, the PPV of NT-proBNP for readmission within 30 days was low; this is unsurprising given that most readmitted patients had a positive NT-proBNP assay (i.e. exceeding the age- and sex-corrected 97.5th percentile), with mean NT-proBNP values lower among subjects readmitted within 30 days compared with non-readmissions. The NPV and PPV for TnI were insignificant for all periods. The addition of TnI to NT-proBNP did not increase its negative-predictive power.
Readmission of patients who developed AHF during the index admission
AHF was observed in increasing frequency for patients readmitted in each subsequent time period, but this trend did not reach statistical significance.

Discussion
The role of biomarkers for predicting AHF during index admission
Elevated TnI at the index ACS event does not predict subsequent development of AHF. This justifies evaluation of alternative biomarkers for predicting AHF. NT-proBNP levels, both on admission and at 6–12 hours after symptoms onset, were significantly higher in patients who developed AHF during the index admission compared with those who did not. This is in keeping with findings by De Lemos et al. who demonstrated higher baseline BNP level among subjects with new or worsening HF within 30 days of ACS.9
Both NT-proBNP and TnI had a high NPV for development of AHF during the index admission. Our data support a role for either of the serum markers in identifying those patients at low risk of developing AHF.
The role of biomarkers for predicting readmission to hospital after ACS event
NT-proBNP concentrations were lower for patients readmitted within 30 days than for those who were not. For every other period the inverse was true. Given that LVSD is reflected by elevated NT-proBNP, only late readmissions may be reasonably attributed to LVSD and HF. Indeed, the higher prevalence of underlying IHD among the late readmission group would reasonably increase these patients’ susceptibility to LVSD following ACS. By contrast, early readmissions were more likely to have suffered UA (as opposed to STEMI), hence, extensive infarction and the LVSD associated with it is less likely in this group.15,16 The underlying cause for early readmissions is probably multi-factorial and has been described by Keith et al.15 For our cohort, potential reasons include initial burden of infarction, patient factors and complications of ACS treatment. Higher incidence of readmission following complications of ACS treatment has been reported previously.16
For all periods, TnI levels were significantly lower among readmissions. The PPV and NPV of TnI for readmission were low for all periods. The NPV of NT-proBNP for readmission was reduced by the addition of TnI. This is an interesting new finding given the focus of recent research into high-sensitivity troponin assays.
The NPV of NT-proBNP for readmission within 30 days exceeded 90% and may play a role in excluding readmission for lower-risk patients. However, a positive assay does not correlate with an increased risk. The value of NT-proBNP for a risk-stratification tool in NSTEMI has been advocated by Eggers et al.,17 who found it independently predictive of clinical outcome up to six months, and superior to TnI and C-reactive protein. Indeed, the ESC has labeled NT-proBNP the most useful ‘rule-out test’ for HF.18
Our finding that a greater NT-proBNP value among readmissions beyond 30 days did not reach statistical significance may reflect our small sample size. A larger multi-centre study with a longer follow-up period presents a next step.
Our study highlights the unreliability of using single markers (even troponin) to predict outcomes for ACS patients. This has been documented in the international GRACE (Global Registry of Acute Coronary Events) study.19 Our results support using NT-proBNP as part of overall risk assessment; the NPV of NT-proBNP for adverse outcome is high and superior to TnI. However, given the poor PPV of NT-proBNP for predicting AHF and readmission, research into alternative markers of adverse outcome for this patient population is indicated.
Conflict of interest
None declared.
Key messages
- Despite progress in recognition of acute coronary syndrome (ACS) and its short- and long-term management, ACS is still associated with a significant burden of post-acute mortality and morbidity, including acute heart failure (AHF) and readmission
- Development of AHF and, therefore, left ventricular systolic dysfunction (LVSD) may predispose to readmission
- Tropinin I (TnI) is an unreliable marker of development of AHF and hospital readmission and does not add to the predictive value of N-terminal pro-brain natriuretic peptide (NT-proBNP) when the two biomarkers are used in combination
- NT-proBNP has a high negative-predictive value (NPV) as a ‘rule out’ test for both end points, though its positive-predictive value (PPV) is low

References
1. Fox KAA, Carruthers KF, Dunbar DR et al. Underestimated and under-recognized. The late consequences of acute coronary syndrome. Eur Heart J 2010;31:2755–64. http://dx.doi.org/10.1093/eurheartj/ehq326
2. Butcher L. Partners against penalties. Trustee 2013;66:24–6.
3. Zdravkovic V, Mladenovic V, Colic M et al. NT-proBNP for prognostic and diagnostic evaluation in patients with acute coronary syndromes. Kardiol Pol 2013;71:472–9. http://dx.doi.org/10.5603/KP.2013.0093
4. Morrow DA, de Lemos JA, Sabatine MS et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non–ST-elevation myocardial infarction. B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol 2003;41:1264–72. http://dx.doi.org/10.1016/S0735-1097(03)00168-2
5. Omland T, de Lemos JA, Morrow DA et al. Prognostic value of N-terminal pro-atrial and pro-brain natriuretic peptide in patients with acute coronary syndromes. Am J Cardiol 2002;89:463–5. http://dx.doi.org/10.1016/S0002-9149(01)02271-8
6. Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators. N-terminal proB-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004;110:3206–12. http://dx.doi.org/10.1161/01.CIR.0000147611.92021.2B
7. Choy AM, Darbar D, Lang CC et al. Detection of left ventricular dysfunction after acute myocardial infarction: comparison of clinical echocardiographic and neurohormonal methods. Br Heart J 1994;72:16–22. http://dx.doi.org/10.1136/hrt.72.1.16
8. Omland T, Aakvaag A, Bonarjee VV et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996;93:1946–50. http://dx.doi.org/10.1161/01.CIR.93.11.1963
9. de Lemos JA, Morrow DA, Bentley JH et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001;345:1014–20. http://dx.doi.org/10.1056/NEJMoa011053
10. Richards AM, Nicholls MG, Yandle TG et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin. New neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation 1998;97:1921–9. http://dx.doi.org/10.1161/01.CIR.97.19.1921
11. Valli N, Gobinet A, Bordenave L. Review of 10 years of the clinical use of brain natriuretic peptide in cardiology. J Lab Clin Med 1999;134:437–44. http://dx.doi.org/10.1016/S0022-2143(99)90163-4
12. Hamm CM, Bassard JP, Agewall S et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. http://dx.doi.org/10.1093/eurheartj/ehr236
13. Steg Ph G, James SK, Atar D et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–619. http://dx.doi.org/10.1093/eurheartj/ehs215
14. Dickstein K, Cohen-Solal A, Filippatos G et al. ECS guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2008;29:2388–442. http://dx.doi.org/10.1093/eurheartj/ehn309
15. Keith KD, Bock JJ, Kobemick MS, Krome RL, Ross MA. Emergency department revisits. Ann Emerg Med 1989;18:964–8. http://dx.doi.org/10.1016/S0196-0644(89)80461-5
16. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty day hospital readmissions following acute myocardial infarction: a community study. Ann Intern Med 2012;157:11–18. http://dx.doi.org/10.7326/0003-4819-157-1-201207030-00004
17. Eggers KM, Lagerqvist B, Venge P, Wallentin l, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2009;54:357–64. http://dx.doi.org/10.1016/j.jacc.2009.03.056
18. Remme WJ, Swedberg K. The European Society of Cardiology Task Force Report. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527–60. http://dx.doi.org/10.1053/euhj.2001.2783
19. Fox KAA, Eagle KA, Steg Ph G, Anderson FA. The global registry of acute coronary events 1999 to 2009 – GRACE. Heart 2010;96:1095–101. http://dx.doi.org/10.1136/hrt.2009.190827
20. Lang RM, Bierig M, Devreux RB et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. http://dx.doi.org/10.1016/j.echo.2005.10.005