In a previous issue of the BJC, key issues regarding the use of high-sensitivity troponin and its use in clinical context were raised.1 Despite the clear benefits with regards to earlier identification of ‘troponin-positive patients’, it is vital to highlight that troponin is specific for myocardial injury, but is not specific for the diagnosis of acute myocardial infarction (MI). Echocardiography is increasingly being used in cases where a ‘troponin-positive event’ is out of keeping with the history and examination for a type I MI. Competent use of this imaging modality can have drastic alterations in the management of patients and potentially prevent invasive cardiological procedures that may later provide more risk than benefit. This case report highlights the caution we must take when requesting troponin biomarkers and the use of echocardiography to aid in the management of the haemodynamically unstable patient.
Case report
A 72-year-old man with a background of hypertension presented to the Accident and Emergency (A&E) department with diarrhoea and vomiting for the past two weeks. With the help of a collateral history from the patient’s wife, it unfolded that the patient had taken to his bed and was not eating or drinking very much. She stated that, on the day prior to his admission, she went upstairs to check up on her husband and found him on the floor. Fortunately, he was fully alert and had not sustained any injury. The patient had no recollection of the ‘fall’ and stated he may have lost consciousness for a brief period. The patient denied any symptoms prior to the fall but stated he was much more short of breath than usual, which he had put down to his presenting complaint.
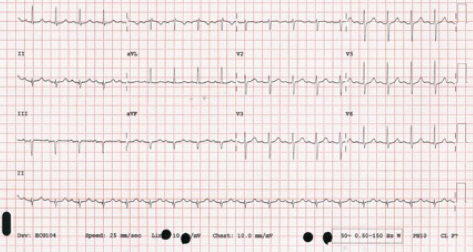
This patient was admitted on to the Acute Coronary Unit due to the need for a side room and remained stable over night with no requirement for medical review. His observations through the night showed a blood pressure (BP) of 130/80 mmHg, pulse rate of 102 bpm, respiratory rate of 16 and pulse oximetry of 95% on room air. His blood tests were generally unremarkable and a 12-lead electrocardiogram (ECG) did not indicate any cause for concern (figure 1).
The patient, however, had a 12-hour troponin I of 0.09 µg/L (0.07 µg/L being the upper limit of normal). Based on this result, the patient was given 300 mg of aspirin and clopidogrel and started on treatment dose enoxaparin (1 mg/kg twice daily). A referral was then made to the cardiology department the following morning, after the medical post-take ward round, with a view to further management of this patient’s ‘NSTEMI’ (non-ST-elevation MI).
On revisiting this patient’s history, he had never suffered from any exertional chest pain, led a healthy and active lifestyle, including being a member of the local ‘ramblers club’. On examination he had a loud second heart sound but nothing else of note. On fully removing the bed sheet, he had a swollen right lower leg, but this was not tender or erythematous. Later that day, the patient dropped his BP to 88/70 mmHg and was fluid resuscitated.
Based on the findings, there was no indication that this patient had suffered a type I MI (defined as an event related to atherosclerotic plaque rupture, ulceration, fissuring, erosion, or dissection with resulting intraluminal thrombus in one or more of the coronary arteries, leading to decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis)2 and, therefore, his acute coronary syndrome (ACS) treatment was stopped and an echocardiogram was requested to elicit any regional wall motion abnormality (RWMA) and view the right heart.
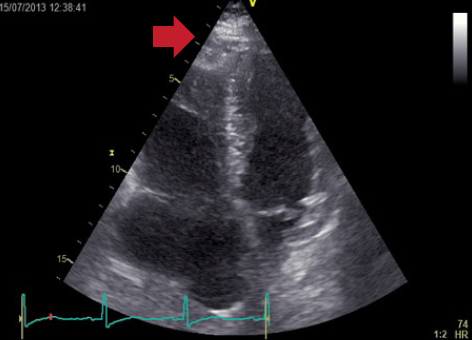
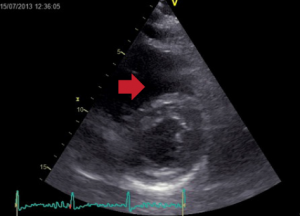
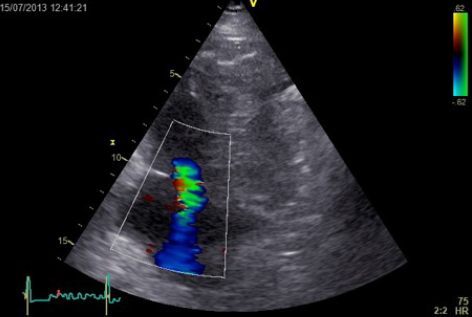
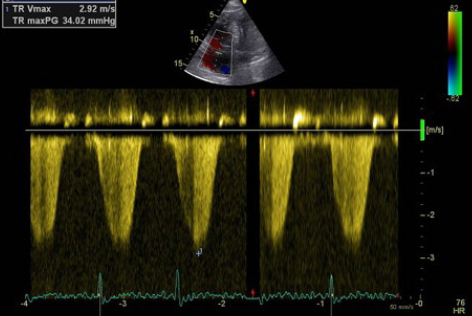
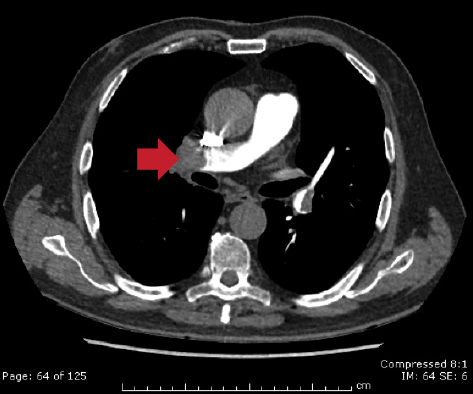
Echocardiography did not reveal RWMA but highlighted typical features of ‘right heart strain’ (figure 2). Recommendation was made to the medical team caring for the patient to consider a computed tomography (CT) pulmonary angiogram (CTPA) and that the troponin was a reflection of the myocardial injury sustained through a secondary cause. The CTPA showed bilateral large segmental pulmonary emboli (figure 3). The patient was started on warfarin and subsequently discharged to continue his journey with his ‘fellow ramblers’.

Discussion
Increased applications of transthoracic (TTE) and transoesophageal (TOE) echocardiography (echo) along with the availability of portable machines, has made this imaging technique even more recognisable in clinical practice. We are increasingly using echocardiography to ‘shine light’ on diagnostic uncertainties where certain investigations throw open differential diagnoses not initially considered.
The imaging gold standard of ‘ruling in’ pulmonary embolism (PE) is a CTPA and, in those patients with suspected massive pulmonary embolus (MPE), should be completed within one hour of presentation.3 The emergence of multi-detector CTPA has offered substantially improved scanning speed and resolution with reported sensitivity and specificity for PE of above 90%.4 However, CTPA can take time to be requested, conducted, yield results and cannot be performed instantly at the patient’s bedside.
This brings echocardiography into play. Such an imaging modality, in rare cases, is able to directly visualise a thrombus in the pulmonary artery or right heart chambers. However, it is the more subtle changes seen with this imaging tool that can significantly change the course of management for patients. Dilatation of the right heart chambers, abnormal inter-ventricular septal contraction and increased systolic pulmonary artery pressure may be observed.5 Unfortunately, these findings are uncommon and too infrequently elicited. Kasper et al. (1986) confirmed these limitations in 105 patients with known PE.
In their study, right ventricular dilation was found in 70 (75%) patients, reduced left ventricular diameter in 38 (42%) patients, dilated right pulmonary artery in 78 (77%) patients, and abnormal motion of the septum in 41 (44%) patients. 6
Additionally, they concluded that, although adding echocardiography to the diagnostic strategy for PE would avoid about 12% to 28% of lung-scan angiography procedures, it would cause inappropriate treatment in 4% to 14% of patients.6 These restrictions are confirmed by Bova et al. (2003), who found that, from a cohort of 162 patients with suspected PE, the sensitivity and specificity of TTE ranged between 29% and 52% and 96% and 87%, respectively.7 Kearon (2003) also concluded that transthoracic echocardiography visualises intracardiac thrombi (usually right atrium) in about 5% of patients with acute pulmonary embolism, and generally does not detect emboli in the pulmonary arteries.8
However, the use of TTE and TOE in haemodynamically unstable patients shows promising results. Nazeyrollas et al. (1996) examined 70 patients admitted to the cardiac intensive care unit with a clinical suspicion of PE: 31 patients had PE confirmed on pulmonary angiography while 39 patients had negative scans. Of those individuals with a confirmed diagnosis of PE, TTE showed significant differences in several echocardiographic variables compared with those with an absence of PE. These included right ventricular diameter >25 mm (70% vs. 14%), ratio of right ventricular diameter to left ventricular diameter >0.5 (85% vs. 22%), and tricuspid velocity >2.5 m/s (93% vs. 18%).9 This correlated to 93% sensitivity for these echocardiographic parameters in PE with haemodynamic compromise, compared with 67% in those with stable haemodynamic parameters.10 TOE equally shows improvements in diagnostic reliability when used to investigate patients with suspected MPE. Krivec et al. (1998) noted that 13 out of 24 patients admitted to the medical intensive care unit with unexplained shock and distended neck veins had evidence of central pulmonary emboli on TOE. The sensitivity and specificity of TOE for PE in patients with right ventricular dilation confirmed on TTE was 92% and 100%, respectively.11 Additionally, Wittlich et al. (1992) noted sensitivity 97% and specificity 88% for TOE in detecting central emboli when compared with ventilation-perfusion scan and pulmonary angiography.12
As a stand-alone imaging technique for the diagnosis of PE, echocardiography is clearly too insensitive when compared with CTPA. However, for acute, haemodynamically compromised patients, echocardiography is attractive since it is a non-invasive bedside investigation and does not require the transport of an unstable patient. Additionally, in those patients who are haemodynamically compromised from an underlying PE, 80% of patients will show signs of right ventricular (RV) dysfunction on echo.13 However, the differential diagnosis of RV dysfunction is broad and includes a number of processes that can cause both RV dilation and dysfunction. Thus, findings on echocardiography should be considered in the clinical context of the patient. This is especially important as with the patient in this report who developed haemodynamic instability and a raised cardiac troponin (cTn).
In their current guidelines, the Joint European Society of Cardiology/American College of Cardiology committee redefined acute MI as an elevation of cTn in blood above the 99th percentile of a healthy reference population in conjunction with signs or symptoms of ischaemia.14 Simply, an isolated raised cTn is not sufficient to diagnose ACS, and there are a myriad of other potential diseases causing troponin release. For example, Spies et al. (1998) found that among patients who were treated in intensive care units for sepsis or systemic inflammatory response syndrome (SIRS), elevated cTn was detected in 85% of patients (cTnI >0.1 µg/ml).15
Furthermore, individuals with chronic stable heart failure (HF)16 showed elevated troponin I (cTnI) values in 15–23% of cases (>0.1 µg/ml) and in acute HF 52–55% of cases.17 Willging et al. (1998) has shown that up to 19% of haemodialysis patients with end-stage renal failure (ESRF) had elevated cTnI.18
However, could PE cause this patent’s cTn rise? In acute PE, elevated cTn is found in up to 50% of cases.19 For cTnT, rates of 32% of patients with a serum cTnT of 0.1 µg/ml were reported.20 Furthermore, among 38 patients with confirmed PE,21 elevated cTnI values of >0.4 µg/ml were seen in 48% of the cohort group. Unfortunately, the reasons why cTn appear in the blood after PE remain unclear. It is primarily believed that cTn are released from injured right ventricular myocardial cells due to the acute dilatation of the right ventricle as a consequence of the abrupt increase of pulmonary artery pressure.22 In the study by Meyer et al. (2000), 63% of those with right ventricular dilation had an increased cTnI level, whereas 29% of positive cTnI levels had a normal right ventricular end-diastolic diameter.23 Another explanation to an elevated troponin in PE patients might be hypoxaemia due to perfusion–ventilation mismatch, hypoperfusion as a consequence of low output and reduced coronary blood flow, as well as paradoxical embolism from systemic veins to the coronary arteries, usually via a patent foramen ovale. Interestingly, a positive cTnI level correlated with having more segmental defects on ventilation–perfusion scintigraphy suggesting a different mechanism to right ventricular strain as a cause for an elevated cTnI in PE.23 Furthermore, Coma-Canella et al. (1988) highlighted the finding that transmural right ventricle infarction, despite patent coronary arteries, was discovered in autopsies of patients who died of massive PE.24
Studies investigating the release kinetics of cTnT in patients with PE showed that the peak cTnT was lower and persisted for a shorter time compared with cTnT values in acute MI. Hence, in contrast to ACS, where cTn are released only after irreversible myocardial damage, in patients with PE, the brief appearance of small amounts of cTn suggests that troponin elevation may be caused by the efflux of the free cytosolic pool of cTnT due to transient membrane leakage.25 Additionally, several studies have reported an association between elevated troponin levels and a poor prognosis in patients with PE. In a study of 577 patients with a confirmed diagnosis of PE, 32% of the cohort had an elevated cTnT of >0.01 µg/L, and it was shown that cTnT was significantly and independently associated with in-hospital and long-term mortality whether analysed as a continuous or categorical variable (p<0.001).26 Furthermore, a recent meta-analysis focused on normotensive patients with acute symptomatic PE. In this analysis, consisting of 1,366 patients, elevated troponin level resulted in a four-fold increased risk of short-term death.27
In conclusion, this case shows that we must always consider troponin in the context of our patient and, although troponin assays can be argued to give prognostic information, they should not be used as a ‘stand alone marker’ for type I MI. In addition, we have highlighted one of the many uses of echocardiography that can aid in the management of non-cardiac pathology •
Sources and selection criteria
We looked at recent conference proceedings and searched PubMed, the Cochrane Database of Systematic Reviews, and Clinical Evidence online using the terms “echocardiography and pulmonary embolism”, “troponin and pulmonary embolism”, and “echocardiography and shock”.
Acknowledgement
The authors would like to thank Dr Elias, Consultant Radiologist, for the CT pulmonary angiography images and reports.
Conflict of interest
None declared.
Key messages
- The importance of cautious interpretation of an elevated troponin in the context of the patient’s clinical presentation
- The advantages and disadvantages of echocardiography in the diagnosis of pulmonary embolus
- The use of bedside echocardiography in the haemodynamically unstable patient
- Common echocardiographic changes associated with a massive pulmonary embolus
References
1. Gamble JHP, Carlton E, Orr W, Greaves K. High sensitivity troponin: six lessons and a reading. Br J Cardiol 2013;20:109–12. http://dx.doi.org/10.5837/bjc.2013.026
2. Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. http://dx.doi.org/10.1093/eurheartj/ehs184
3. British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470–83. http://dx.doi.org/10.1136/thorax.58.6.470
4. Aghajanzadeh D, Gassanov N, Schmidt M et al. Imaging techniques for the diagnosis of pulmonary embolism. Reports in Medical Imaging 2010;3:129–39. http://dx.doi.org/10.2147/RMI.S11623
5. Augusseau-Richard MP, Pacouret G, Dessenne X, Charbonnier B. Echocardiography in pulmonary embolism. Arch Mal Coeur Vaiss 1995;88(11 suppl):1715–22.
6. Kasper W, Meinertz T, Henkel B et al. Echocardiographic findings in patients with proved pulmonary embolism. Am Heart J 1986;112:1284–90. http://dx.doi.org/10.1016/0002-8703(86)90361-3
7. Bova C, Greco F, Misuraca G et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003;21:180–3. http://dx.doi.org/10.1016/S0735-6757(02)42257-7
8. Kearon C. Diagnosis of pulmonary embolism. CMAJ 2003;168:183–94. Available from: http://www.cmaj.ca/content/168/2/183.long
9. Nazeyrollas P, Metz D, Jolly D et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism.Eur Heart J 1996;17:779–86. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014946
10. Perrier A, Tamm C, Unger P-F, Lerch R, Sztajzel J. Diagnostic accuracy of Doppler echocardiography in unselected patients with suspected pulmonary embolism.Int J Cardiol 1998;65:101–09. http://dx.doi.org/10.1016/S0167-5273(98)00107-7
11. Krivec B, Voga G, Zuran I et al. Diagnosis and treatment of shock due to massive pulmonary embolism.Chest 1997;112:1310–16. http://dx.doi.org/10.1378/chest.112.5.1310
12. Wittlich N, Erbel R, Eichler A et al. Detection of central pulmonary artery thromboemboli by transesophageal echocardiography in patients with severe pulmonary embolism. J Am Soc Echocardiogr 1992;5:515–24. http://dx.doi.org/10.1016/S0894-7317(14)80043-6
13. Cohen R, Loarte P, Navarro V, Mirrer B. Echocardiographic findings in pulmonary embolism: an important guide for the management of the patient.World J Cardiovasc Dis 2012;2:161–4. http://dx.doi.org/10.4236/wjcd.2012.23027
14. Antman E, Bassand J-P, Klein W et al. Myocardial infarction redefined – a consensus document of the Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–69. http://dx.doi.org/10.1016/S0735-1097(00)00804-4
15. Spies C, Haude V, Overbeck M et al. Serum cardiac troponin T as a prognostic marker in early sepsis. Chest 1998;113:1055–63. http://dx.doi.org/10.1378/chest.113.4.1055
16. Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation 1997;96:2953–8. http://dx.doi.org/10.1161/01.CIR.96.9.2953
17. Perna ER, Macin SM, Parras JI et al. Cardiac troponin T levels are associated with poor short- and long-term prognosis in patients with acute cardiogenic pulmonary edema. Am Heart J 2002;143:814–20. http://dx.doi.org/10.1067/mhj.2002.120152
18. Willging S, Keller F, Steinbach G. Specificity of cardiac troponins I and T in renal disease. Clin Chem Lab Med 1998;36:87–92. http://dx.doi.org/10.1515/CCLM.1998.016
19. Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart 2006;92:987–93. http://dx.doi.org/10.1136/hrt.2005.071282
20. Giannitsis E, Muller-Bardorff M, Kurowski V et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000;102:211–17. http://dx.doi.org/10.1161/01.CIR.102.2.211
21. Mehta NJ, Jani K, Khan IA. Clinical usefulness and prognostic value of elevated cardiac troponin I levels in acute pulmonary embolism. Am Heart J 2003;145:821–5. http://dx.doi.org/10.1016/S0002-8703(02)94704-6
22. Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J 2011;32:404–11. http://dx.doi.org/10.1093/eurheartj/ehq456
23. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol 2000;36:1632–6. http://dx.doi.org/10.1016/S0735-1097(00)00905-0
24. Coma-Canella I, Gamallo C, Martinex Onsurbe P, Lopez-Sendon J. Acute right ventricular infarction secondary to massive pulmonary embolism. Eur Heart J 1988;9:534–40. Available from: http://eurheartj.oxfordjournals.org/content/9/5/534.abstract
25. Muller-Bardorff M, Weidtmann B, Giannitsis E, Kurowski V, Katus HA. Release kinetics of cardiac troponin T in survivors of confirmed severe pulmonary embolism. Clin Chem 2002;48:673–5. Available from: http://www.clinchem.org/content/48/4/673.long
26. Ng ACC, Yong ASC, Chow V et al. Cardiac troponin-T and the prediction of acute and long-term mortality after acute pulmonary embolism. Int J Cardiol 2013;165:126–33. http://dx.doi.org/10.1016/j.ijcard.2011.07.107
27. Jiménez D, Uresandi F, Otero R et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest 2009;136:974–82. http://dx.doi.org/10.1378/chest.09-0608
Further reading
Sosland RP, Gupta K. McConnell’s sign. Circulation 2008;118:e517–e518. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.746602
Nixdorf U, Erbel R, Drexler M, Meyer J. Detection of thromboembolus of the right pulmonary artery by transesophageal two-dimensional echocardiography. Am J Cardiol 1988;61:488–9. http://dx.doi.org/10.1016/0002-9149(88)90320-7
Pruszczyk P, Torbicki A, Kuch-Wocial A et al. Transesophageal echocardiography for definitive diagnosis of haemodynamically significant pulmonary embolism.Eur Heart J 1995;16:534–8. Available from: http://eurheartj.oxfordjournals.org/content/16/4/534.abstract
Pruszczyk P, Torbicki A, Pacho R et al. Noninvasive diagnosis of suspected severe pulmonary embolism: transesophageal echocardiography vs spiral CT.Chest 1997;112:722–8. http://dx.doi.org/10.1378/chest.112.3.722
Vieillard-Baron A, Qanadli SD, Antakly Y et al. Transesophageal echocardiography for the diagnosis of pulmonary embolism with acute cor pulmonale: a comparison with radiological procedures. Intensive Care Med 1998;24:429–33. http://dx.doi.org/10.1007/s001340050591
Ginsberg JS, Wells PS, Kearon C et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism. Ann Intern Med 1998;129:1006–11. http://dx.doi.org/10.7326/0003-4819-129-12-199812150-00003

