The latest mega trial − IMPROVE-IT − dominated discussion at the recent American Heart Association (AHA) Scientific Sessions, with ezetimibe becoming the first non-statin lipid lowering agent to reduce clinical events. Other major talking points at the meeting, held in Chicago on 15th−19th November 2014, were the optimal duration for dual antiplatelet therapy after drug-eluting stent placement, whether oxygen may be harmful for STEMI patients who are not hypoxic, and more doubts about aspirin in primary prevention.
IMPROVE-IT: ezetimibe reduces events on top of simvastatin
 The long-awaited IMPROVE-IT trial has shown that adding ezetimibe to treatment with simvastatin lowers the risk of future cardiovascular events in high-risk patients who had recently suffered an acute coronary syndrome (ACS).
The long-awaited IMPROVE-IT trial has shown that adding ezetimibe to treatment with simvastatin lowers the risk of future cardiovascular events in high-risk patients who had recently suffered an acute coronary syndrome (ACS).
The addition of ezetimibe to simvastatin 40 mg was associated with a low-density lipoprotein (LDL) lowering of 15 mg/dL (0.4 mmol/L) which translated into a 6.4% lower risk of cardiovascular events.
Lead investigator, Dr Christopher Cannon (Brigham and Women’s Hospital, Boston, USA) said: “The study is the first to show that adding a non-statin drug to a statin to improve cholesterol levels can help patients do better. We took those patients from a clinically appropriate target LDL to even lower. We now have solid evidence that lower is good, and even lower can be even better.”
IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) enrolled 18,144 patients within 10 days of an ACS with LDL levels less than 125 mg/d (3.2 mmol/L) or less than 100 mg/dl (2.59 mmol/L) if they were already on a statin. They were randomised to simvastatin 40 mg or to simvastatin 40 mg plus ezetimibe 10 mg.
Average baseline LDL level was 95 mg/dl (2.45mmol/L). This was lowered to 69.5 mg/dl (1.8 mmol/L) in the simvastatin alone group and 53.7 mg/dL (1.39 mmol/L) in the simvastatin plus ezetimibe group.
The trial was event driven and ran for seven years. Results showed a 6.4% reduction in the primary end point of cardiovascular death, myocardial infarction (MI), unstable angina, coronary revascularisation, or stroke (table 1). The number needed to treat to prevent one event was 50.
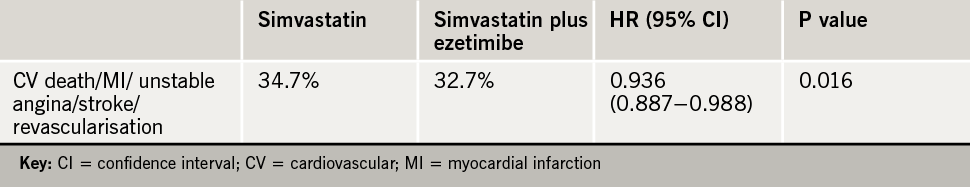
Benefits were seen for the individual end points of MI (14% reduction), and stroke (14% reduction). There was no difference in cardiovascular deaths between the two groups.
Safety data was reassuring, with no statistically significant differences in any adverse event with ezetimibe including cancer or muscle- or gallbladder-related events.
Noting that atorvastatin 80 mg is now the most popular treatment for ACS patients, Dr Cannon said he thought the IMPROVE-IT results could be extrapolated to this situation, and that it was now reasonable to start ACS patients on atorvastatin 80 mg plus ezetimibe right from the start. Others might be happier starting on atorvastatin 80 mg and then adding in ezetimibe later on if LDL levels are not at goal, he added.
Commenting on the study, Dr Lori Mosca (Director of Preventive Cardiology at New York-Presbyterian Hospital, USA) said: “These results will help expand our treatment options for high-risk ACS patients, especially among those who are intolerant of or who do not achieve desired results with intense statin therapy These results are consistent with decades of research in high-risk ACS patients affirming the central role of aggressive LDL reduction in the prevention of recurrent heart disease. They further suggest that we should consider setting the LDL bar even lower among our high-risk patients to achieve maximum benefit to prevent recurrent heart disease and stroke.”

Giving a UK perspective, Professor Anthony Wierzbicki (Guys and St Thomas Hospital, London) commented to the BJC: “This will have to be reviewed by NICE, but what we can say is that we have a positive trial result and a safe drug. All the argument will be where it is best used from a cost-effectiveness view.” He suggested that it would be likely deemed cost-effective in the high-risk ACS group, but maybe not in lower risk chronic disease.
He added: “Everything will change of course when ezetimibe comes off patent in 2017–2018. There will be far wider usage then. Until then there are bound to be some restrictions in the UK. But these will be a lot less prescriptive than they have been.”
Online extra
More in-depth analysis of this landmark study can be found at www.bjcardio.co.uk. We also talk to Professor Wierzbicki about the implications of this study for practice in our podcast online.
Optimal dual antiplatelet therapy after stenting: no easy answer
The question as to how long dual antiplatelet therapy should be continued after placement of a drug-eluting stent was addressed in three new studies presented at the AHA meeting.
Unfortunately the results did not appear, at first look at least, to be consistent. Experts concluded that dual antiplatelet therapy has to be individualised. Six months treatment may be sufficient for low-risk patients receiving one of the latest third-generation drug-eluting stents, but high-risk patients (such as those with ACS) may require treatment for 30 months or even longer, maybe for life.
Trying to draw conclusions from the plethora of new data presented Dr Giles Montalescot (Pitié-Salpêtrière University Hospital, Paris, France) said the issue of how long to continue dual antiplatelet therapy should be thought of as two separate scenarios − short-term treatment after stenting to prevent stent thrombosis, and long-term treatment of higher risk patients as secondary prevention for native coronary heart disease.
He concluded: “There is no magic number for the duration of dual antiplatelet therapy. I suggest that the new data presented here show that if the patient has had a new-generation stent and you want to stop early at six months you probably can. But if the patient has multiple ischaemic factors, you would want to continue for a long time, maybe forever.”
The three new trials presented were the large DAPT (American Dual Antiplatelet Therapy) study, and two smaller European trials – ISAR-SAFE (Intracoronary Stenting and Antithrombotic Regimen: Safety and Efficacy of Six Months Dual Antiplatelet Therapy After Drug-Eluting Stenting) and ITALIC (Is There a Life for Drug-Eluting Stents after Clopidogrel).
DAPT
With almost 10,000 patients, the DAPT trial is the largest study to look at this issue and was designed in conjunction with the US Food and Drug Administration to answer the question of optimal treatment time for dual antiplatelet therapy. After 12 months of dual therapy, patients were randomised to continue with clopidogrel or prasugrel (on top of aspirin) for an additional 30 months or to stop thienopyridine treatment and continue on aspirin alone. Patients who had already had an event or a serious bleed in the first 12 months were excluded.
Results (table 1) showed that patients who continued on dual therapy were almost four times less likely to develop in-stent thrombosis and had about half the risk of having new heart attacks than patients who stopped thienopyridine. This was at the cost of an increase in GUSTO moderate bleeding (1.7% vs. 1.0%), but severe bleeding was not increased.
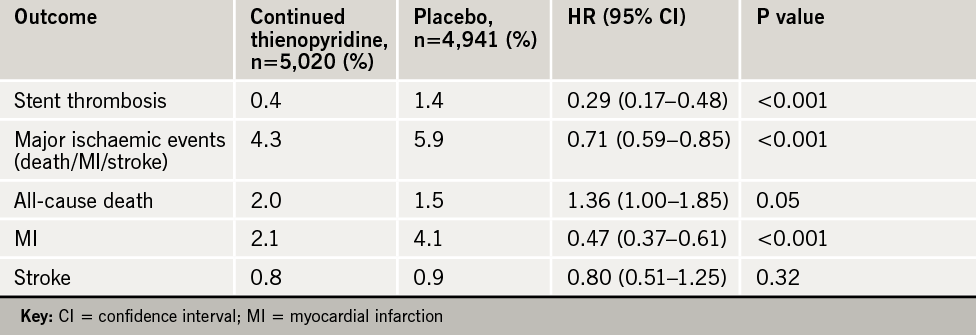
The DAPT trial showed a hazard of stopping treatment, with a marked increase in ischaemic events in the three-month period after discontinuing clopidogrel or prasugrel whenever that occurred. This led to suggestions that treatment should continue longer than 30 months, maybe even for life.
Noting that half the myocardial infarctions (MIs) prevented were not related to stent thrombosis, lead investigator, Dr Laura Mauri (Brigham and Women’s Hospital, Boston, USA) suggested that longer duration treatment was having a general secondary-prevention effect.
There was also a puzzling increase in all-cause death in the continued treatment group, and a secondary analysis revealed that this appeared to be attributable to trauma and cancer.
Dr Mauri said: “It appears that there may have been an imbalance between the groups in the number of patients with known cancer before enrollment in the study.” To provide additional reassurance on this, the DAPT researchers conducted a meta-analysis of 14 studies in a total of almost 70,000 patients randomised to shorter- or longer-term dual antiplatelet therapy. This found that compared with aspirin alone or short-duration dual antiplatelet therapy (<6 months), continued treatment was not associated with a difference in all-cause mortality.
Dr Mauri concluded: “Continued thienopyridine therapy is beneficial in patients who have tolerated one year of dual antiplatelet therapy after drug-eluting-stent placement. These results should be applied excluding patients with a history of major bleeding.”
ISAR-SAFE/ITALIC
The ISAR-SAFE trial, presented by Dr Stefanie Schulz-Schüpke (Deutsches Herzzentrum, München, Germany) randomised 4,000 patients to six or 12 months of clopidogrel after drug-eluting-stent implantation; all patients were taking aspirin. The trial was stopped early because of a low event rate. Results showed no difference between the two treatment groups in ischaemic events or major bleeding.
Similar results were seen in the ITALIC trial, presented by Dr Martine Gilard (University of Brest, France). In this study, 2,031 patients who received the Xience V™ (Abbott Laboratories) drug-eluting stent were randomised to receive six- or 24-month dual antiplatelet therapy. Again stopped early because of a low event rate, the study also showed no significant difference between the two groups regarding the primary end point − a composite of death, MI, urgent target vessel revascularisation, stroke, and major bleeding at 12 months.
Both Drs Shultz and Gilard concluded that six months of dual therapy was a “realistic option”, especially in patients a low risk of future events.
In his discussion of the studies, Dr Montalescot pointed out that the ISAR-SAFE and ITALIC trials were conducted in Europe where third-generation drug-eluting stents are more widely available. “These studies showed very low event rates which suggests we can safely stop dual therapy at six months with these stents if the patient is at low overall risk or has risk factors for bleeding,” he concluded.
The DAPT trial was published in the New England Journal of Medicine, the meta-analysis in The Lancet, and the ITALIC trial in Journal of the American College of Cardiology, all online on 16th November 2014.
ASPIRIN − no benefit in Japanese primary prevention study
Daily low-dose aspirin had no significant benefit in the primary prevention of cardiovascular events in patients with multiple risk factors in a new Japanese study.
The event rate in the study was much lower than expected, so the possibility that aspirin does have a beneficial effect in this population cannot be excluded, “but its benefit was still less than anticipated in this population,” lead investigator, Dr Kazuyuki Shimada (Shin-Oyama City Hospital, Tochigi, Japan) concluded.
JPPP (The Japanese Primary Prevention Project) randomised 14,658 patients with hypertension, dyslipidaemia, and/or diabetes mellitus without a history of cardiovascular disease to 100 mg of enteric-coated aspirin per day or no aspirin.
After a median of five years follow-up, the study was prematurely terminated due to futility. Results showed a non-significant 6% reduction in the risk of a primary end point event in the aspirin group on the composite end point of cardiovascular death/myocardial infarction (MI)/stroke. There were significant reductions in MI and in transient ischaemic attack, but a significant increase in serious extracranial haemorrhage (hazard ratio 1.85), and an increase in gastrointestinal adverse events.
Discussant of the study, Dr Dorairaj Prabhakaran (London School of Hygiene and Tropical Medicine) said it was difficult to show a benefit of aspirin when given on a background of widespread statin use. “Benefit is very unlikely in low-risk populations such as those with less than 1% events per year, but there could be a role in higher risk groups and in low-/middle-income countries like India, where the risk of coronary artery disease is extremely high.”
The study was published online in JAMA on 17th November 2014. An accompanying editorial notes that more data will come from the ongoing ASCEND, ARRIVE, and ASPREE trials, and additional information on the cancer benefits of aspirin will help decision making.
Oxygen harmful in STEMI if patient not hypoxic?
Giving ST-elevation myocardial infarction (STEMI) patients supplemental oxygen may be doing more harm than good if they are not hypoxic, a new study suggests. The AVOID (Air Versus Oxygen in ST-Elevation Myocardial Infarction) trial compared supplemental oxygen to no oxygen unless oxygen levels fell below 94%.
In the study, STEMI patients were randomised to receive oxygen prehospital and right through the angioplasty procedure until they were stable on the ward (n=218), or to receive no oxygen unless they became hypoxic (n=223).
Results showed a significant 25% increase in creatine kinase (CK) and a non-significant increase in troponin levels in those given oxygen. “This suggests increased myocardial injury in those delivered oxygen,” said lead investigator Dr Dion Stub (Baker IDI Heart and Diabetes Institute, Melbourne, Australia).
20-year WOSCOPS results: statin benefits remain
20-year follow up of patients in the WOSCOPS (West of Scotland Primary Prevention Study) has shown that the benefit of five years of statin use remains all those years later.
The WOSCOPS trial involved 6,595 men who were randomised to 40 mg/day of pravastatin or placebo. Published in 1995, it showed a 31% reduced risk of cardiovascular death/myocardial infarction.
For the long-term follow-up, Dr Chris Packard (University of Glasgow) and colleagues examined linked death registers and hospitalisation records.
They found that patients in the original pravastatin arm had a 27% reduced risk of coronary heart disease mortality, and a 13% reduced risk of all-cause mortality.
Dr Packard noted that the 20 year follow up period covered the age range from 55 to 75 years. “This is when premature cardiovascular disease occurs.”
The results also supported the long-term safety of statins as non-cardiovascular events and cancers showed similar incidence rates in the two groups.
Online extra
More from this study can be found at www.bjcardio.co.uk
ODYSSEY ALTERNATIVE: alirocumab for statin intolerance
Many patients who were thought to be statin intolerant actually managed to stay on low-dose atorvastatin in the ODYSSEY ALTERNATIVE study, but for those who cannot tolerate a statin, the new PCSK9 antibodies look like a good alternative.
The study enrolled 361 patients with statin intolerance documented in their medical history. Mean baseline low-density lipoprotein was 190 mg/dl (4.91 mmol/L).
The sensitivity of this patient population was demonstrated by the fact that 47 patients dropped out during the four-week placebo run-in.
The remaining 314 patients were randomised to the new PCSK9 antibody, alirocumab 75/150 mg by subcutaneous injection every two weeks (n=126), ezetimibe 10 mg once daily (n=125) or atorvastatin 20 mg once daily (n=63) for 24 weeks. Then all patients continued on open label alirocumab.
Amazingly, 75% of patients labeled as statin intolerant could actually tolerate the 20 mg dose of atorvastatin.
During the blinded treatment phase, 23% of alirocumab patients stopped treatment, versus 33% in both other treatment arms. LDL levels were reduced by 45% in the alirocumab group, 14% in the ezetimibe group and 20% in the atorvastatin patients.
Adverse effects showed the vast majority of patients reported some adverse events with each of the three treatments, but skeletal muscle adverse events and side-effects necessitating drug discontinuation appeared less in the alirocumab group (table 1).
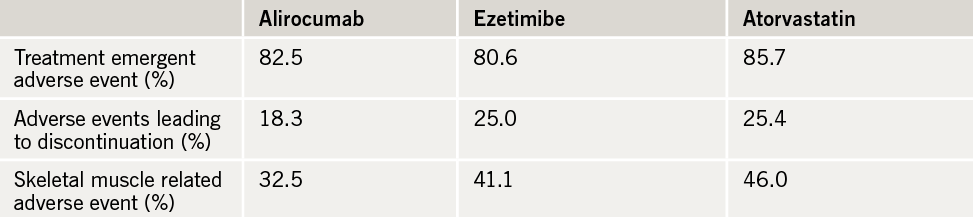
The adverse event rate was lower during the open label alirocumab treatment phase, with just 3% of patients discontinuing the therapy because of adverse events.

Presenting the study, Dr Patrick Moriarty (University of Kansas Medical Center, USA), reported that 10–25% of patients in clinical practice report statin intolerance. “Knowing how to treat these patients is very difficult as large, well-controlled randomised trials of cholesterol-lowering drugs in statin intolerant patients are lacking.” He added that these patients are notoriously difficult to treat, often experiencing adverse effects on most other drugs too, even placebo.
Online extra
More analysis of this important study and five other studies reporting in the ODYSSEY programme can be found at www.bjcardio.co.uk. We also talk to Professor Robin Choudhury (Oxford University) about the implications of this study for practice, particularly the diagnosis of statin intolerance, in our podcast available online only.
PARAMEDIC: mechanical no better than manual chest compression for CPR
 Using mechanical devices to perform consistent chest compressions during cardiopulmonary resuscitation (CPR) efforts does not improve survival compared to manual chest compressions in people who have had a non-trauma related cardiac arrest outside hospital, according to results of the PARAMEDIC study.
Using mechanical devices to perform consistent chest compressions during cardiopulmonary resuscitation (CPR) efforts does not improve survival compared to manual chest compressions in people who have had a non-trauma related cardiac arrest outside hospital, according to results of the PARAMEDIC study.
The study included 4,471 patients treated by four ambulance services in the UK who were randomised to manual compressions (2,819) or mechanical compressions delivered by the LUCAS-2® portable, electrically-powered device.
Results found that 30-day survival was similar after mechanical (6.3%) and manual (6.9%) compressions. There was also no difference in the percentage of patients who survived to reach hospital, in whom pulse and breathing was restored or whose brain function after the event was good enough to allow them to live independently.
Lead investigator, Dr Gavin Perkins (University of Warwick) said that while the device did not improve survival it still had practical advantages. “These include performing safe and quality CPR in an emergency situation which can free up a pair of hands to attend to other tasks.”
The study was published in The Lancet online on 16th November 2014 to coincide with its presentation. An accompanying editorial notes that results from real-world studies with mechanical devices have suggested higher rates of return to spontaneous circulation and survival to discharge, but results from three randomised trials have not shown a significant survival benefit. It points out that these trials are difficult to perform and that a definite answer may still not be known. While mechanical CPR is more costly, it allows ambulance crews to be safely belted up and is “a logical choice from the safety perspective,” it adds.
Other studies examined risk of internal injuries from mechanical chest compression devices and found no difference in serious or life-threatening injuries to organs in the chest or abdomen between the LUCAS® device and manual compressions. But a different device − the Autopulse® (which delivers compressions from a band wrapped around the chest) was associated with a higher rate of organ damage than manual compressions (11.6% vs. 6.6%).
Unnecessary aspirin use in patients on oral anticoagulants
Many patients with atrial fibrillation (AF) who are on oral anticoagulants are also taking aspirin or other antiplatelet medication unnecessarily, a new Canadian registry study suggests.
The study, part of the SPRINT-AF (Stroke Prevention and Rhythm Interventions in AF) registry, analysed data from 936 patients with non-valvular AF of whom 88% were taking oral anticoagulants. Of those on anticoagulation, 18.2% were also treated with an antiplatelet agent, but 40% of these patients had no valid indication for antiplatelet therapy.
Presenting the study, Dr Milan Gupta (McMaster University, Hamilton, Canada) explained that 60% of patients who received antiplatelet therapy had a clinical indication such as a history of coronary, cerebrovascular, or peripheral arterial disease. Even in these patients, it is unknown whether antiplatelet theapy is still needed if they are also taking oral anticoagulation for AF. “But we do know that the combination of antiplatelet and anticoagulants will increase bleeding risk substantially,” he said.
Endocarditis cases rising in England after antibiotic ban
The number of cases of infective endocarditis have risen in England following a decision in 2008 to stop recommending prophylactic antibiotics before dental surgery, a new study shows.
But Professor Martin Thornhill (University of Sheffield School of Clinical Dentistry) cautioned that they had not demonstrated a cause and effect relationship, and there may be other explanations for the change.
The National Institute for Health and Care Excellence (NICE) is reviewing the study and the guidelines, and has issued a press release urging practitioners not to change their practice until they concluded their review.
Until 2008, antibiotic prophylaxis had been recommended for patients at high or moderate risk of endocarditis in the UK who were undergoing dental surgery.
The researchers identified all prescriptions of a single oral dose of amoxicillin 3 g or clindamycin 600 mg (the recommended prophylaxis prior to invasive dental procedures) that were dispensed in England from 2004 to 2013. They found that prior to the 2008 change in guidelines, clinicians wrote 10,900 prescriptions a month for single doses of these drugs, with 90% being issued by dentists.
After the change in the guidelines, the prescription rate fell exponentially, and by March 2013, the average number of prescriptions had fallen by almost 90% to 1,307 a month.
During the same period, the number of cases of endocarditis per month rose by 0.11 cases per 10 million people, and by March 2013 this amounted to an extra 35 cases per month. There were 420 additional hospitalisations and 18 additional deaths related to endocarditis each year.
The study was published online in The Lancet on 18th November 2014. An accompanying commentary calls for a randomised study to ascertain whether prophylactic antibiotics are needed.
Trans fats linked to worse memory
The consumption of trans fats was linked to worse memory among healthy working-age men in a new study.
The study, which involved 1,000 men, showed that those who consumed the most trans fats had notably worse performance on a word memory test. The association remained even after controlling for many factors including age, education, ethnicity and depression.
Lead author, Dr Beatrice Golomb (University of California-San Diego, USA) commented: “From a health standpoint, trans fat consumption has been linked to higher body weight, more aggression and heart disease. As I tell patients, while trans fats increase the shelf life of foods, they reduce the shelf life of people.”
