A 26-year-old man presented to the emergency department with central chest pain radiating to the left arm. An electrocardiogram (ECG) revealed inferior ST elevation. He had no major risk factors for cardiovascular disease, but habitually used synthetic cannabinoids (AKB48 and 5F-AKB48). A subsequent coronary angiogram showed occlusions in four obtuse marginal branches of the left circumflex artery and a large clot in the distal right coronary artery. The patient was treated with aspirin, ticagrelor, rivaroxaban and tirofiban infusion, and these occlusions were demonstrated to have resolved on a follow-up angiogram. The patient admitted smoking 5F-AKB48 four hours before the onset of chest pain. This case further strengthens the association between the use of synthetic cannabinoids and embolic-appearing myocardial infarction. This is the first report of myocardial infarction associated with the currently ‘legal-high’ 5F-AKB48, and may indicate the need for tighter regulation of this compound.
Introduction
In recent years, the recreational use of synthetic cannabinoids has been gaining global popularity.1-5 Case reports have emerged associating these compounds with a number of adverse effects, including: embolic-appearing ischaemic strokes,6 seizures7 and acute kidney injury.8 In addition, myocardial infarction (MI) has been associated with synthetic cannabinoid use in teenagers.9,10 However, no cases have demonstrated abnormal coronary angiography.
There are numerous synthetic cannabinoids, including JWH-018, JWH-073, HU-210, CP 47,497, JWH-081, JWH-122, JWH-210, and newer compounds are regularly being developed.4 A proportion of these cannabimimetics have been classified as Schedule One substances in the USA,11 and some have been classified as Class B drugs in the UK.12 However, legislation has struggled to keep pace with the new generations of subtly altered cannabimimetics. Several synthetic cannabinoids, including 5F-AKB48, are yet to be classified in the UK and are currently available as ‘legal highs’. These compounds are available over-the-counter, often from retailers of smoking paraphernalia and via the internet. Street names for these drugs include: spice, clockwork orange, black mamba, K2, exodus and cyclone.4,7
Here, we present a case of an embolic-appearing MI with multiple distal vessel occlusions apparently related to synthetic cannabinoid use. Due to the association of synthetic cannabinoid use with this potentially devastating pattern of MI, we urge stricter control of these substances.
Case presentation
A 26-year-old man presented with sudden onset central chest pain radiating to his left arm. He was woken by the pain and taken to his local emergency department by ambulance. He described the pain as ‘crushing’ in nature and 9/10 in severity. The pain was associated with shortness of breath and not relieved by sublingual glyceryl trinitrate spray. The electrocardiogram (ECG) in the ambulance revealed lateral ST-depression and, in the emergency department, a second ECG showed inferior ST-elevation. On examination the patient was cold peripherally, sweaty and clammy. Blood pressure, temperature, heart rate, oxygen saturation and an arterial blood gas were unremarkable. Jugular venous pressure was not clinically elevated, heart sounds were normal and the chest was clear.
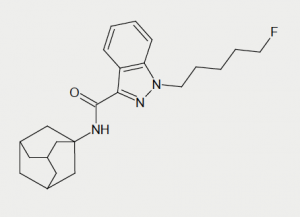
The patient admitted a history of recreational drug use. The only recreational drug he reported taking in the last six weeks was the synthetic cannabinoid 5F-AKB48 (figure 1). His last dose was at midnight, four hours prior to the onset of his chest pain. The patient reported cutting down on smoking the drug during the previous six weeks as his friend had suffered a seizure when smoking 5F-AKB48 for the first time. Before this, the patient was a heavy smoker of synthetic cannabinoids, mainly AKB48 and its fluoro derivative. He purchased 5F-AKB48 as a powder over the internet, dissolved it in a solvent and used it to impregnate tobacco leaves, which were then smoked. The patient consumed around 2500 mg of synthetic cannabinoid per week and had smoked almost every day for the past three years.
In the month prior to admission, the patient reported episodes of exertional chest pain, which coincided with initiating propranolol (40 mg four-times daily) for anxiety. This chest pain was similar in character to, but less severe than the pain suffered on the day of admission. His only other regular medication was mirtazapine 45 mg once daily. The patient reported past use of recreational illicit drugs including cocaine and heroin four years ago.
The patient had a history of anxiety-related disorders, including agoraphobia and social phobia. He lives with his mother and is unemployed.
Investigations
Troponin I rose from <0.09 to 1.47 µg/L on admission, implying a damaged myocardium. Urea and electrolytes, full blood count, lipids and liver function tests were normal; blood cultures were negative. Therefore, the patient was transferred by ambulance to a tertiary centre for a diagnostic angiogram.
Urine toxicology was negative for amphetamines, benzodiazepines, cocaine, methadone, and cannabis. A psychoactive drug screen was also negative. Urine toxicology was positive only for morphine, given to the patient in the ambulance, the emergency department and on the ward. At the time of the patient’s admission no laboratory tests were available to detect synthetic cannabinoids.
Markers of hypercoagulability and immune dysfunction were all unremarkable (cardiolipin antibodies, beta 2 microglobulin, antiB2 glycoprotein antibodies, antithrombin III, protein C, protein S, activated partial thromboplastin time, fibrinogen, prothrombin time, lupus anticoagulant, factor V leiden, serum electrophoresis, antinuclear antibodies, extractable nuclear antigen screen, rheumatoid factor, glomerular basement membrane antibodies, antimitochondrial antibodies, smooth muscle antibodies, liver-kidney microsomal antibodies, antineutrophil cytoplasmic antibodies, complement C3, complement C4).
Echocardiogram showed mild left ventricular dysfunction with hypokinesis/akinesis of all segments of the left ventricular walls at the apex (figure 2).
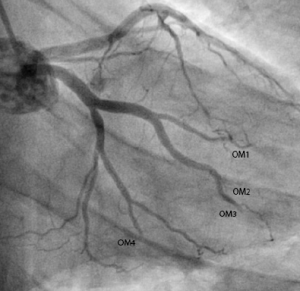
Diagnostic angiogram revealed minor coronary artery disease with occlusions in four of the obtuse marginal branches (OM1, OM2, OM3 and OM4) of the left circumflex artery (figure 3). Furthermore, a large clot was visualised in the distal right coronary artery and a filling defect noted in the posterior descending artery (PDA) (figure 4).
Treatment
The decision was taken to treat with pharmacology rather than with percutaneous intervention. The patient was treated aggressively with antiplatelet agents; aspirin 75 mg once daily, 90 mg ticagrelor twice daily and tirofiban infusion (25 µg/kg intravenous infused over three minutes and subsequently 0.15 µg/kg/min) for 72 hours. He was then converted to aspirin 75 mg once daily, clopidogrel 75 mg once daily and rivaroxaban 5 mg once daily.
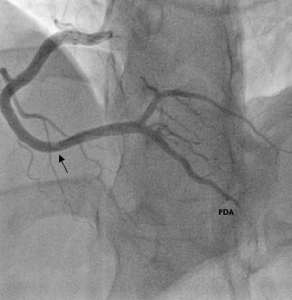
Post-treatment angiogram shows almost complete resolution of the filling defects (figures 5 and 6).
Discussion
MI in young people without major cardiovascular risk factors, and MI involving multiple distal vessels, are both rare occurrences. We associate this unusual pattern of MI with this patient’s use of synthetic cannabinoids. The patient reports no recent use of any other recreational or illicit substances (supported by negative toxicology screening). There are a growing number of reports associating synthetic cannabinoid use with embolic-appearing phenomena in young people.4,6,10
To our knowledge this is the first case report of synthetic cannabinoid-associated MI with:
- angiographic evidence of coronary occlusion
- involving the compound 5F-AKB48.
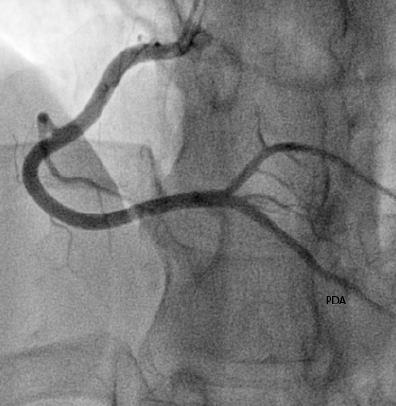
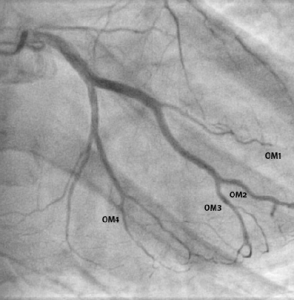
Further, we demonstrate resolution of coronary occlusions with aggressive antiplatelet and anticoagulation therapy (aspirin, ticagrelor, tirofiban and rivaroxaban).
The mechanism of the infarction appears to be embolic. However, the previous symptoms of similar chest pains on starting propranolol may indicate a possible vasospastic component.
Also, the infarction following the relatively recent change to the fluoro derivative of AKB48 is interesting. This may prolong the compound’s half-life and/or promote platelet aggregation, as is seen with some fluorinated prostaglandin analogues.13
One limitation of this report is that no methods were locally available to confirm presence of synthetic cannabinoids in biological samples. However, an association of synthetic cannabinoids and their purported adverse effects would be worth investigating as methods of detecting synthetic cannabinoids become more widely available.11,14-16
Despite association with the potentially devastating effect of MI, only a proportion of synthetic cannabinoids are classified as Class B drugs in the UK.12 Many, including 5F-AKB48, remain legally available and are colloquially considered ‘legal highs’. Structural modification of synthetic cannabinoids has resulted in the rapid production of new compounds, outpacing the current legal regulatory processes.4,17 Broad regulation may be inappropriate since it might limit the development of some of these compounds for therapeutic purposes.18,19 However, in light of the associated adverse effects, we urge more robust restrictions on the supply and distribution of synthetic cannabinoids for recreational purposes.
In view of the growing popularity of the recreational use of synthetic cannabinoids, doctors should be aware of their association with MI and other adverse effects, including seizures, stroke and acute kidney injury. They should also be aware of local capabilities in testing for these emerging compounds.
Conflict of interest
None declared.
Editors’ note
See also the editorial by Russo in this issue.
Key messages
- Recreational drug history, including ‘legal-highs,’ is an important component of chest pain clerking in the young
- Synthetic cannabinoids are associated with a variety of adverse effects
- Myocardial infarctions associated with 5F-AKB48 may show atypical angiograms
- Occluded coronary vessels can be resolved with aggressive antiplatelet and anticoagulation therapy (aspirin, tirofiban, clopidogrel and rivaroxaban)
- There is an increasing need for tighter regulation of synthetic cannabinoids
References
1. Caviness CM, Tzilos G, Anderson BJ, Stein MD. Synthetic cannabinoids: use and predictors in a community sample of young adults. Subst Abus 2014;published online. http://dx.doi.org/10.1080/08897077.2014.959151
2. Curtis B, Alanis-Hirsch K, Kaynak O, Cacciola J, Meyers K, McLellan AT. Using web searches to track interest in synthetic cannabinoids (aka ‘herbal incense’). Drug Alcohol Rev 2014;published online. http://dx.doi.org/10.1111/dar.12189
3. Papaseit E, Farre M, Schifano F, Torrens M. Emerging drugs in Europe. Curr Opin Psychiatry 2014;27:243–50. http://dx.doi.org/10.1097/YCO.0000000000000071
4. Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 2012;39:234–43. http://dx.doi.org/10.1016/j.pnpbp.2012.04.017
5. Winstock AR, Barratt MJ. The 12-month prevalence and nature of adverse experiences resulting in emergency medical presentations associated with the use of synthetic cannabinoid products. Hum Psychopharmacol 2013;28:390–3. http://dx.doi.org/10.1002/hup.2292
6. Freeman MJ, Rose DZ, Myers MA, Gooch CL, Bozeman AC, Burgin WS. Ischemic stroke after use of the synthetic marijuana “spice”. Neurology 2013;81:2090–3. http://dx.doi.org/10.1212/01.wnl.0000437297.05570.a2
7. de Havenon A, Chin B, Thomas KC, Afra P. The secret “spice”: an undetectable toxic cause of seizure. Neurohospitalist 2011;1(4):182–6. http://dx.doi.org/ 10.1177/1941874411417977
8. Thornton SL, Wood C, Friesen MW, Gerona RR. Synthetic cannabinoid use associated with acute kidney injury. Clin Toxicol 2013;51:189–90. http://dx.doi.org/10.3109/15563650.2013.770870
9. McKeever RG, Vearrier D, Jacobs D, LaSala G, Okaneku J, Greenberg MI. K2-not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: a case report. J Med Toxicol 2014;published online. http://dx.doi.org/10.1007/s13181-014-0424-1
10. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 2011;128:e1622–e1627. http://dx.doi.org/10.1542/peds.2010-3823
11. Elsohly MA, Gul W, Wanas AS, Radwan MM. Synthetic cannabinoids: analysis and metabolites. Life Sci 2014;97:78–90. http://dx.doi.org/10.1016/j.lfs.2013.12.212
12. UK Government. The Misuse of Drugs Act 1971 (Amendment) Order 2013. Available from: http://www.legislation.gov.uk/uksi/2013/239/contents/made
13. Lakin KM, Makarov VA, Kovalev SG et al. [The effect of fluorinated prostaglandins on platelet aggregation]. Eksp Klin Farmakol 1994;57:39–41.
14. Mazzarino M, de la Torre X, Botre F. A liquid chromatography-mass spectrometry method based on class characteristic fragmentation pathways to detect the class of indole-derivative synthetic cannabinoids in biological samples. Anal Chim Acta 2014;837:70–82. http://dx.doi.org/10.1016/j.aca.2014.06.003
15. Rodrigues WC, Catbagan P, Rana S, Wang G, Moore C. Detection of synthetic cannabinoids in oral fluid using ELISA and LC-MS-MS. J Anal Toxicol 2013;37:526–33. http://dx.doi.org/10.1093/jat/bkt067
16. Spinelli E, Barnes AJ, Young S et al. Performance characteristics of an ELISA screening assay for urinary synthetic cannabinoids. Drug Test Anal 2014;published online. http://dx.doi.org/10.1002/dta.1702
17. Brents LK, Prather PL. The K2/spice phenomenon: emergence, identification, legislation and metabolic characterization of synthetic cannabinoids in herbal incense products. Drug Metab Rev 2014;46:72–85. http://dx.doi.org/10.3109/03602532.2013.839700
18. Dando I, Donadelli M, Costanzo C et al. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis 2013;4:e664. http://dx.doi.org/10.1038/cddis.2013.151
19. Ramer R, Fischer S, Haustein M, Manda K, Hinz B. Cannabinoids inhibit angiogenic capacities of endothelial cells via release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. Biochem Pharmacol 2014;91:202–16. http://dx.doi.org/10.1016/j.bcp.2014.06.017
