In June 2014 the National Institute for Health and Care Excellence (NICE) released new guidelines (TA314) on the use of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy devices (CRTs) in the management of heart failure. These guidelines replaced the previous TA95 and TA120 guidelines. We evaluated the potential impact on implant rates in our institution.
Clinical records of 396 consecutive patients were reviewed, with 100 patients included in the final analysis. Device indications and associated costs were calculated using both existing and new criteria.
NICE TA95/TA120 criteria recommended 37 devices: 20 ICDs, 9 CRTs with pacing (CRT-Ps), and 8 CRTs with defibrillator (CRT-Ds). The new NICE 2014 criteria recommended 97 devices: 56 ICDs, 7 CRT-Ps, and 34 CRT-Ds. Comparison of the new and old guidelines suggested a significant increase in total devices (p<0.0001). This corresponded primarily to an increase in ICDs and CRT-Ds, with an associated £661,708 increase in total spend (£407,205 increase per annum).
This study confirms the significant increase in ICDs and CRT-Ds indicated by NICE. This will have significant financial and workforce implications.
Introduction
Heart failure is a complex syndrome that places a significant burden on the National Health Service (NHS). The UK incidence of heart failure is 140 per 100,000 men and 120 per 100,000 women per annum, with approximately 900,000 people in England and Wales affected by the syndrome.1 It accounts for one million inpatient bed-days (2% of the NHS total) and 5% of all emergency hospital admissions.2
Implantable cardiac electronic devices (ICEDs) have revolutionised the management of heart failure in patients with a reduced left ventricular ejection fraction (LVEF), already on optimal medical therapy. Implantable cardioverter defibrillators (ICDs) effectively treat life-threatening ventricular arrhythmias, which account for up to 50% of mortality in heart failure with reduced LVEF.3 In addition, cardiac resynchronisation therapy (CRT) improves symptoms, reduces hospitalisation, and prolongs life in patients with heart failure and a broad QRS complex on electrocardiography (ECG).4
However, the provision of ICEDs has a significant impact on the local and national health economy. In June 2014, the National Institute for Health and Care Excellence (NICE) released new guidelines (TA314)1 (table 1) regarding such devices, updating previous guidelines released in January 2006 (TA95) and May 2007 (TA120).5,6 We investigated the potential impact of NICE TA314 on ICED implant rates in our institution.

Methods
The clinical records of 396 consecutive patients admitted to our hospital and referred to the heart failure service between 1 January 2013 and 15 August 2014 were reviewed. The following parameters were recorded: date of birth, sex, admission date, New York Heart Association (NYHA) class on admission, LVEF, ECG rhythm and QRS duration (including presence of bundle branch block), presence of ischaemic heart disease, pre-existing ICED therapy, and if death occurred. Exclusion criteria were: death during admission, presence of an existing ICD or CRT, and absence of a recorded LVEF.
Where no numerical LVEF was recorded in an echocardiogram report, the following was applied, as per British Society of Echocardiography guidance: mild, moderate and severe left ventricular systolic dysfunction corresponded to LVEF values of 45–55%, 35–45% and <35%, respectively. Where patients were admitted more than once in the study period, data corresponding to the admission with the most severe LVEF and QRS duration values were used.
Patients with a LVEF ≤35% were included in the analysis, with their NYHA class at follow-up after a period of pharmacological optimisation collected from records. ICED eligibility was calculated according to the following criteria (figure 1):
- NICE 2006/2007: indications using NICE TA95 criteria, relaxed to include patients with non-ischaemic aetiologies of heart failure, and NICE TA120 criteria, relaxed to include patients with atrial fibrillation, as per local policies.
- NICE 2014: indications using a common interpretation of NICE TA314 criteria; patients with a LVEF of ≤35%, NYHA class I to III, and QRS duration <120 ms are deemed by many cardiologists to be high risk, and therefore eligible for an ICD.

When applying NICE 2006 criteria, patients with a 24-hour tape positive for non-sustained ventricular tachycardia (NSVT) were deemed eligible for an ICD, despite not undergoing electrophysiological studies, due to their limited use in this scenario. Patients who fulfilled the criteria for 24-hour Holter monitoring, but who had not undergone this investigation, were classified as not having NSVT. When applying NICE 2007 criteria, mechanical dyssynchrony was assessed through an overall assessment of ventricular wall synchrony on echocardiography. When applying NICE 2014 criteria, where both CRT-P (CRT with pacing) and CRT-D (CRT with defibrillator) were stated as suitable options, CRT-D was selected to simplify the analysis.
For the cost analysis (figure 2), prices quoted in the NICE TA314 for ICD (£9,692), CRT-P (£3,411) and CRT-D (£12,293) systems were used. These prices corresponded to the average selling prices aggregated across all manufacturers of the respective systems sold in the UK in 2011.1 Official predictions of the impact of TA314 on device implantation in our institution were available on request from NICE.7

Statistical analysis
Normally distributed demographic data are presented as a mean with standard deviation (SD). Non-normally distributed data are presented as a median with interquartile range (IQR). Categorical data are presented as a count with proportions. Two-way comparisons of NICE criteria were performed using Fisher’s exact test. A p value of <0.05 was considered to be statistically significant.
Results
During the study period, 396 patients were registered with the heart failure service. Of these, 48 patients died during their admission, 20 had an existing ICD or CRT, and 36 had no recorded LVEF and were excluded from further analysis. There were 292 patients included in the analysis. A Kaplan-Meier survival curve stratified by LVEF was plotted (figure 3). Overall survival at 30 days and one year was 92.5% and 67.5%, respectively.

The records of the 143 patients with a LVEF ≤35% were further considered to determine their outcome post-discharge, following a period of pharmacological optimisation. Of this group, 43 patients received no review following discharge. For the remaining 100 patients, NYHA class and pharmacological regimen at follow-up were obtained (table 2). The median time at follow-up was three months (IQR 1–5).
Device recommendations
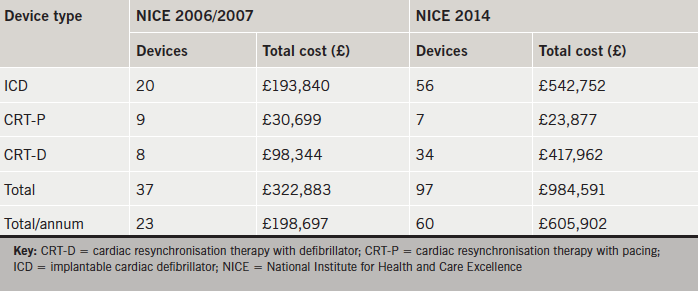
Application of NICE 2006/2007 recommended 37 devices (20 ICDs, nine CRT-Ps and eight CRT-Ds), representing a total cost of £322,883 (£198,697 per annum) (table 3). NICE 2014 recommended 97 devices (56 ICDs, seven CRT-Ps and 34 CRT-Ds), representing a total cost of £984,591 (£605,902 per annum).
Comparison of NICE 2014 with NICE 2006/2007 criteria revealed a 167% relative increase in total devices (p<0.0001). This corresponded primarily to an increase in ICDs (relative increase of 180%) and CRT-Ds (relative increase of 325%).
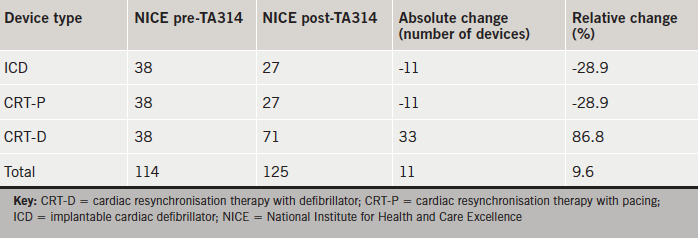
Official predictions from NICE on the impact of TA314 on implant rates in our institution are provided in table 4. In relative terms, ICD and CRT-P numbers were both predicted to decrease by 28.9%. The number of CRT-Ds was predicted to increase by 86.8% with total devices predicted to increase by 9.6%.
Discussion
Summary
Our retrospective analysis suggests NICE TA314 could have significant implications for current practice. A comparison of NICE 2014 with NICE 2006/2007 criteria suggested a highly significant increase in total devices of 167%, because of marked increases in ICD and CRT-D recommendations.
These results suggest that NICE TA314 will significantly increase ICED indications. However, the likely size of this effect depends on the specific interpretation of the guidelines applied. Key questions to consider are: how strictly have NICE 2006/2007 criteria been followed by clinicians? How will clinicians interpret the latest guidelines? NICE 2006/2007 criteria were considered by many as restrictive, due to exclusion of patients with non-ischaemic heart failure aetiologies, and those with atrial fibrillation, from consideration for ICD and CRT devices, respectively. As a result, many institutions, including our own, produced local policies to account for the needs of such patients. For this reason, we have applied this interpretation of the criteria. A significant area of ambiguity in NICE TA314 relates to patients with a LVEF ≤35%, a QRS duration <120 ms and NYHA class between I and III, who can be considered for an ICD if deemed at ‘high risk of sudden cardiac death’. For many clinicians, all patients with a LVEF ≤35% are deemed ‘high risk’ and eligible for a device, and we have chosen to use this interpretation.
NICE predictions
NICE have provided their own estimates for the impact of TA314 on implantation rates in our institution in absolute terms (table 4). Our data suggest that NICE may have underestimated the number of ICDs and CRT-Ds indicated as a result of their latest guidelines, with our study indicating a 167% relative increase in total devices (NICE estimate: 9.6% relative increase). NICE recognise that their predictions are based upon several assumptions. Their pre-TA314 estimates assume current ICD and CRT implant rates per million of 70 and 140, respectively, with an equal split between CRT-Ds and CRT-Ps. Their post-TA314 predictions are based upon a reduction in ICD implant rates to 50 implants per million and an increase in CRT implants to 180 per million (72% of which are CRT-Ds).
Indications for ICD therapy in heart failure
Greater than anticipated increases in device indications have been reported previously following the release of NICE TA95. Plummer and colleagues reported that ICD indications were three times greater than anticipated by NICE.8 Furthermore, the authors suggested that extending the primary prevention criteria to include patients with a history of myocardial infarction and a LVEF ≤30%, regardless of QRS duration or NYHA class, led to a 12-fold increase in ICD indications. These extended criteria stemmed from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) selection criteria.9 A recent survey of 48 European centres found that 80% of centres would implant ICDs in patients with LVEF <35% and QRS duration <120 ms.10 The latest NICE criteria reflect these findings through their consideration of patients with a LVEF ≤35%, a normal QRS duration and a NYHA class between I and III, as eligible for an ICD.
CRT efficacy in heart failure
CRT therapy has been proven to significantly reduce morbidity and mortality in appropriately selected patients with heart failure.11,12 However, with the potential increase in CRT recommendations by NICE, it is important to recognise that almost one-third of patients receiving CRT gain no symptomatic benefit.13 Factors implicated in non-response include gender, aetiology of heart failure, presence of atrial fibrillation, and QRS morphology and duration.14 A better understanding of the mechanisms of non-response is required to guide selection of patients most likely to gain benefit. The increase in CRT-Ds, specifically, may also be met with some scepticism. Looi and colleagues showed that the survival benefits afforded by CRT-Ds over CRT-Ps were attenuated at one-year post-implantation, suggesting that complications other than ventricular tachyarrhythmias were responsible for reduced survival beyond this timepoint.15 This is an important observation given the additional expense associated with CRT-Ds over CRT-Ps.
Financial implications
There is a significant expense associated with ICEDs.16,17 Importantly, the additional 60 devices (37 devices per annum) recommended by comparison of the old and new guidelines corresponded primarily to an increase in ICDs and CRT-Ds, which represent the most expensive systems available. We calculated a £661,708 increase in spend on implantable cardiac devices over the study period (£407,205 increase per annum). However, derivation of the cost impact of NICE TA314 is more complex than simply the cost of ICED systems themselves. Other factors to consider include the costs associated with hospital admission and time in theatre, which must be offset against the cost reductions associated with reduced heart failure-related hospitalisation as a result of ICED therapy. Furthermore, ICED costs, technologies and practices are changing. NICE used average UK prices from 2011 for their analysis, however, such costs are under increasing scrutiny by specialised commissioning. Newer developments, such as quadripolar left ventricular leads, remote ICED monitoring, and the shift to day-case device implantation, all impact on overall costs and cost-effectiveness. A more complex costings analysis that incorporated all of these factors was beyond the remit of this study.
Workforce implications
The increase in the total number of devices suggested by this study would require an increase in the number of cardiologists, nurses and cardiac physiologists to cope with the longer patient list. The implantation of a CRT can take significantly longer than that of an ICD due to the requirement of a left ventricular lead.18 Further considerations would be increased follow-up requirements, including device interrogations and battery changes, and higher numbers of complications associated with these devices.15,19,20
Study population implications
Patient data from admissions prior to the release of NICE TA314 were reviewed as part of our analysis. Patients without a device who were now eligible according to the latest guidelines were referred back to their consultants to discuss device therapy.
Limitations
Patients are required to be on optimal pharmacological therapy before consideration for an ICED. Due to the retrospective nature of this study, a considerable number of patients were lost during follow-up. However, this attrition did not undermine the power of this study due to the highly significant increase in devices recommended by NICE TA314.
A possible contributory factor to the significant increase in devices was poor compliance with certain sections of the old guidelines. For example, 24-hour Holter monitoring was not strictly recommended for all patients with a LVEF ≤35% and NYHA class between I and III. Only a small fraction of this subgroup within our study had 24-hour Holter monitoring (19%). Those without were classified as not having NSVT for our analysis. Another example of poor compliance was the lack of electrophysiological studies and echocardiographic assessments of mechanical dyssynchrony. Lack of adherence to guidelines on ICEDs is certainly not a new issue.10,21 These latest guidelines represent a far simpler and prescriptive iteration that will likely improve clinician compliance.
ICED indications do not always equate to implants for numerous reasons, including patient choice, comorbidities and appropriateness in palliative care. NICE have not provided guidance on such factors, however, clinicians must consider them before recommending an ICED. Clinicians need to be particularly prudent when deciding the appropriateness of defibrillator devices.22,23 As a result, the data in this study likely represent an overestimation of the number of devices indicated by both the old and new guidelines.
Key messages
- The latest National Institute for Health and Care Excellence (NICE) guidelines on the use of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy devices (CRTs) in heart failure may lead to a greater than expected number of device implantations
- In particular, CRT with defibrillator (CRT-D) recommendations are expected to increase
- The predicted increase in device eligibility could have significant financial implications at both a local and national level, due to the costs associated with ICD and CRT-D devices
- An increase in device recommendations could also have implications for workforce planning and training
Acknowledgement
The authors thank the members of the nurse-led heart failure service for their guidance on data collection.
Contributors
MD and OG were involved in the conception and design of the study. TM, OG, DG, and JN were involved in data collection. TM, MD, OG and DM were involved in analysis and interpretation of the data. TM, MD and OG contributed to the writing of the paper as well as developing the structure and arguments of the paper. All authors reviewed and approved the final manuscript.
Conflict of interest
DM has previously received financial support from Medtronic and St. Jude to attend educational meetings. MD has received speaker fees and sponsorship from Medtronic, Sorin, Boston and Biotronik.
Editors’ note
See also the editorial by Dr A Turley in this issue.
References
1. National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120). London: NICE, 2014. Available from: http://guidance.nice.org.uk/ta314
2. National Institute for Health and Care Excellence. CG108. Chronic heart failure: management of chronic heart failure in adults in primary and secondary care. London: NICE, 2010. Available from: https://www.nice.org.uk/guidance/cg108
3. Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol 2010;7:216–25. http://dx.doi.org/10.1038/nrcardio.2010.3
4. Holzmeister J, Leclercq C. Implantable cardioverter defibrillators and cardiac resynchronisation therapy. Lancet 2011;378:722–30. http://dx.doi.org/10.1016/S0140-6736(11)61228-2
5. National Institute for Health and Care Excellence. Technology appraisal guidance 95. Implantable cardioverter defibrillators for arrhythmias. London: NICE, 2006. Available from: https://www.nice.org.uk/guidance/ta95
6. National Institute for Health and Care Excellence. Technology appraisal guidance 120. Cardiac resynchronisation therapy for the treatment of heart failure. London: NICE, 2007. Available from: https://www.nice.org.uk/guidance/ta120
7. National Institute for Health and Care Excellence. Costing template: implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120) TA314. Putting NICE into practice. London: NICE, 2014. Available from: https://www.nice.org.uk/guidance/ta314/costing
8. Plummer CJ, Irving JR, McComb JM. The incidence of implantable cardioverter defibrillator indications in patients admitted to all coronary care units in a single district. Europace 2005;7:266–72. http://dx.doi.org/10.1016/j.eupc.2005.01.006
9. Moss AJ, Zareba W, Hall WJ et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. http://dx.doi.org/10.1056/NEJMoa013474
10. Sciaraffia E, Dagres N, Hernandez-Madrid A, Proclemer A, Todd D, Blomström-Lundqvist C. Do cardiologists follow the European guidelines for cardiac pacing and resynchronization therapy? Results of the European Heart Rhythm Association survey. Europace 2015;17:148–51. http://dx.doi.org/10.1093/europace/euu395
11. Bristow MR, Saxon LA, Boehmer J et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. http://dx.doi.org/10.1056/NEJMoa032423
12. Cleland JGF, Daubert J-C, Erdmann E et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. http://dx.doi.org/10.1056/NEJMoa050496
13. Auricchio A, Prinzen FW. Non-responders to cardiac resynchronization therapy: the magnitude of the problem and the issues. Circ J 2011;75:521–7. http://dx.doi.org/10.1253/circj.CJ-10-1268
14. Kumar P, Schwartz JD. Device therapies: new indications and future directions. Curr Cardiol Rev 2015;11:33–41. http://dx.doi.org/10.2174/1573403X1101141106121553
15. Looi K-L, Gajendragadkar PR, Khan FZ et al. Cardiac resynchronisation therapy: pacemaker versus internal cardioverter-defibrillator in patients with impaired left ventricular function. Heart 2014;100:794–9. http://dx.doi.org/10.1136/heartjnl-2014-305537
16. García-Pérez L, Pinilla-Domínguez P, García-Quintana A et al. Economic evaluations of implantable cardioverter defibrillators: a systematic review. Eur J Health Econ 2014;published online. http://dx.doi.org/10.1007/s10198-014-0637-x
17. Neyt M, Stroobandt S, Obyn C et al. Cost-effectiveness of cardiac resynchronisation therapy for patients with moderate-to-severe heart failure: a lifetime Markov model. BMJ Open 2011;1:e000276. http://dx.doi.org/10.1136/bmjopen-2011-000276
18. León AR, Abraham WT, Curtis AB et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol 2005;46:2348–56. http://dx.doi.org/10.1016/j.jacc.2005.08.031
19. Van Rees JB, de Bie MK, Thijssen J, Borleffs CJW, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995–1000. http://dx.doi.org/10.1016/j.jacc.2011.06.007
20. Chen S, Ling Z, Kiuchi MG, Yin Y, Krucoff MW. The efficacy and safety of cardiac resynchronization therapy combined with implantable cardioverter defibrillator for heart failure: a meta-analysis of 5674 patients. Europace 2013;15:992–1001. http://dx.doi.org/10.1093/europace/eus419
21. Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 2015;17:69–77. http://dx.doi.org/10.1093/europace/euu233
22. Vohra J. Implantable cardioverter defibrillators (ICDs) in octogenarians. Heart Lung Circ 2014;23:213–16. http://dx.doi.org/10.1016/j.hlc.2013.09.010
23. Barra S, Providência R, Paiva L, Heck P, Agarwal S. Implantable cardioverter-defibrillators in the elderly: rationale and specific age-related considerations. Europace 2014;17:174–86. http://dx.doi.org/10.1093/europace/euu296
