Current guidelines make no recommendations regarding the strategy for initiation of oral anticoagulant (OAC) therapy in patients who are hospitalised with newly diagnosed atrial fibrillation (AF). This was a single-centre, retrospective, observational study that included patients admitted in 2013 with newly diagnosed AF (ICD-10 I48). There were 234 patients hospitalised with newly documented AF. The mean CHA2DS2-VASc score was 3.8: 201 (86%) patients had a CHA2DS2-VASc score ≥2. Out of the 179 patients considered for anticoagulation, only 115 patients were intended to receive OAC therapy: 56 (49%) as an inpatient and 59 (51%) as an outpatient, either by anticoagulation clinic or primary care. In the outpatient group, only 41 patients (69%) were actually initiated on OAC, with a mean time delay of 10 and 93 days in anticoagulation clinic and primary care group, respectively. During mean follow-up of 194 days, there were two strokes in the outpatient group in patients intended to start anticoagulation but did not (2/59), while no episodes occurred in the inpatient group.
In summary, only 82% of patients with newly diagnosed AF and CHA2DS2-VASc score ≥2 were referred for initiation of OAC, and still fewer actually received such therapy. Outpatient anticoagulation is associated with poor uptake and significant delays.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia with a reported worldwide prevalence of 0.6% in men and 0.4% in women.1 AF is independently associated with a five-fold increased rate of stroke, which is comparable with the risk seen in patients with three or more other stroke risk factors.2,3 Furthermore, strokes related to AF are associated with higher rates of disability and mortality than other strokes.4-6 The cost of AF-related strokes in the UK is estimated to be around £750 million per year.7
AF-related stroke is thought to be secondary to thromboembolism from the left atrium to the cerebral circulation. Oral anticoagulation (OAC) with warfarin and novel oral anticoagulants (NOACs) reduces the risk of AF-related stroke by about two-thirds.8,9 Clinical guidelines recommend that patients with AF should undergo formal risk stratification for stroke and those without contraindications should be treated with OAC.10
AF is commonly first diagnosed in hospitalised patients. In this patient group, OAC can be initiated either during the inpatient stay or following hospital discharge. There are no published data comparing these two strategies in relation to time to initiation of OAC, length of hospital stay, patient outcomes, or cost. Nor do current clinical guidelines specify the preferred strategy for initiation of OAC in hospitalised patients with newly diagnosed AF.
This study described the strategies used to initiate OAC in hospitalised patients with newly diagnosed AF, and the rate of uptake and time delay in initiating OAC in this group of patients. In addition, we aimed to determine whether there were factors that influenced the strategy used.
Method
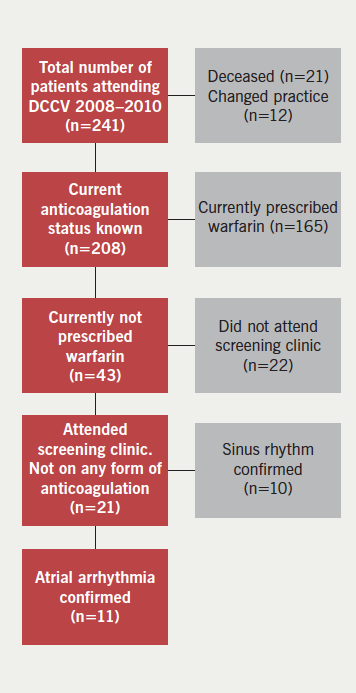
This was a single-centre, observational, cohort study based in a district general hospital in London. The study cohort comprised all patients admitted between 1 January to 31 December 2013 for more than 24 hours, with one of the primary diagnoses being newly documented AF or atrial flutter. Patients were identified retrospectively using discharge diagnosis coding data I48 (AF or atrial flutter) according to the World Health Organization’s International Classification of Diseases 10th revision (ICD-10) coding system. We did not differentiate between AF and atrial flutter because the indications for OAC are the same for both arrhythmias. Figure 1 demonstrates the data collection process. Pre-existing AF was identified from previous discharge diagnosis coding data, paper health records, or from electrocardiogram (ECG) or Holter-monitor reports. In line with current clinical guidelines, we considered that there was a clear indication for OAC in patients whose CHA2DS2-VASc score was ≥2. In the outpatient group, we identified the rate of uptake, time delay and reason(s) for lack of initiation of therapy through directly contacting GPs and anticoagulation clinics. Duration of hospital stay and the specialty team in charge of the patient’s care were recorded. The occurrence of cerebrovascular and major bleeding events during a mean follow-up of around six months after hospital discharge were recorded in all patients. Major bleeding events were defined as bleeding that required invasive intervention such as endoscopic or surgical procedures and/or treatment with blood products.
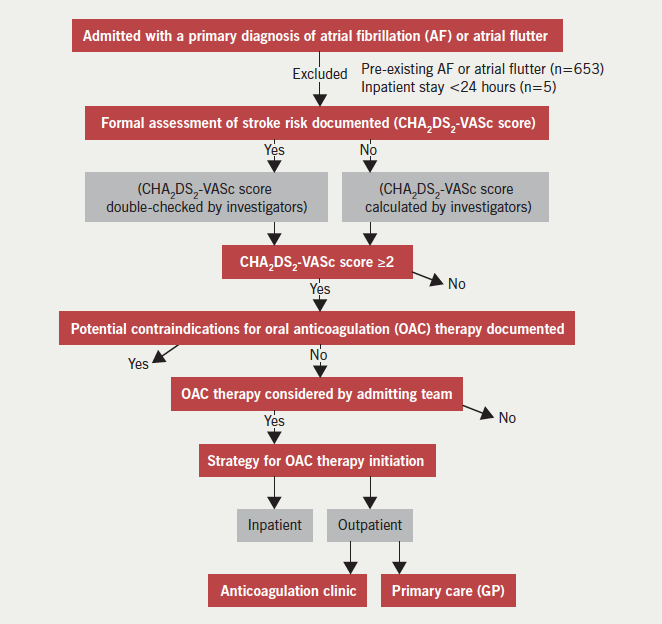
Clinical information was obtained from paper and electronic health records. The following baseline clinical characteristics were recorded: age, sex, hypertension, diabetes mellitus, congestive heart failure (CHF), ischaemic heart disease (IHD), peripheral vascular disease (PVD), and previous stroke. CHF was defined by prior history or imaging evidence of a left ventricular ejection fraction <40%. IHD was defined by a history of angina, myocardial infarction, myocardial revascularisation, or by use of anti-anginals. PVD was defined by self-reported lower limb claudication or a history of lower limb arterial angioplasty or bypass surgery.
Statistical analysis
Continuous variables with a normal distribution were compared using the student t-test, and the Mann Whitney U-test was used to compare non-normal data. Categorical variables were compared using the Z-test. Odds ratios and relative risks were also calculated. All analyses were conducted using SPSS statistical software. A p value <0.05 was considered to be significant. This study was approved as an audit/quality improvement project.
Results
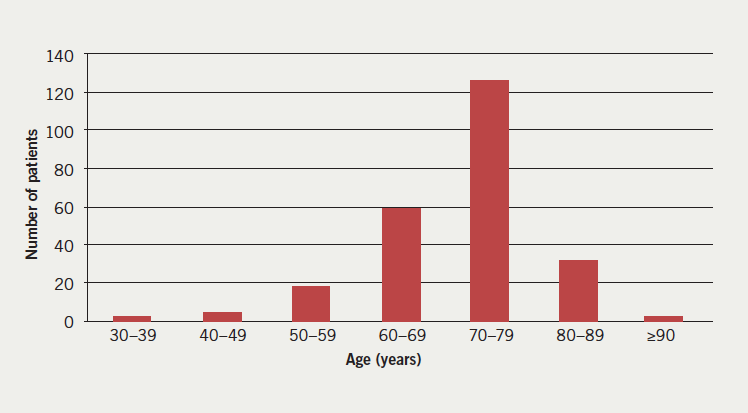
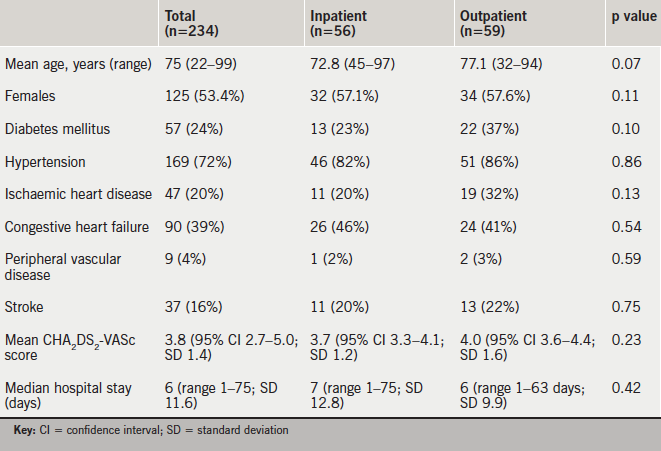
In the one-year study period, 234 patients were newly diagnosed with AF or atrial flutter during a hospital admission >24 hours. Their baseline characteristics are shown in table 1. Two hundred and one (86%) patients had a CHA2DS2-VASc score ≥2, eight patients had a CHA2DS2-VASc score of 1 and 25 patients had a CHA2DS2-VASc score of 0.
Formal assessment of stroke risk
CHA2DS2-VASc scores were documented in the medical records of 202 (86%) of 234 patients. Among the 201 patients in whom we determined that OAC was clearly indicated, CHA2DS2-VASc scores were documented in 179. In the remaining 22 patients, there was no documentation to suggest that stroke risk had been assessed or that OAC therapy had been considered. Our retrospective assessment of stroke risk showed that these patients had a mean CHA2DS2-VASc score of 3.8. None of these patients were commenced on OAC therapy. Neither were any of the 33 patients with a CHA2DS2-VASc score ≤1.
Initiation of oral anticoagulation
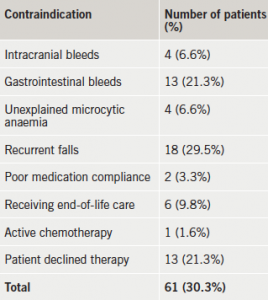
Among the 179 patients who were considered for OAC therapy, 61 had documented contraindications or declined therapy (table 2). Three further patients were not anticoagulated (inappropriately) because of the paroxysmal nature of their AF. The remaining 115 patients were intended to receive OAC: 56 were initiated as an inpatient (49%) and 59 (51%) were referred for outpatient initiation (table 1).
Those with CHA2DS2-VASc scores above the mean (≥4) were not more likely to have inpatient OAC compared with those below the mean (odds ratio [OR] 0.72; 95% confidence interval [CI] 0.39–1.34; p=0.31). Cardiologist compared with non-cardiologist was not more likely to use inpatient initiation (OR 1.08; 95% CI 0.58–2.00; p=0.81), however, they were more likely to utilise anticoagulation clinics rather than GPs for outpatient OAC (OR 13.67; 95% CI 6.14–30.38; p<0.0001).
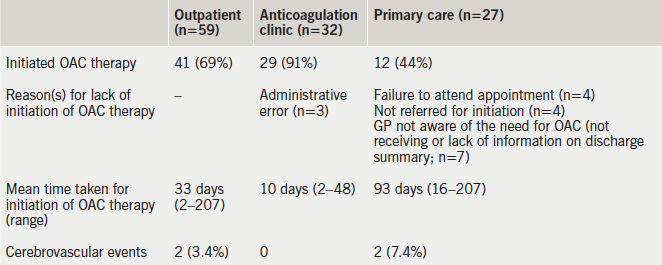
Initiation of OAC as an outpatient was associated with poor uptake, with 18 patients failing to commence on therapy, and mean delays of 33 days as a whole, and 93 days in the primary care arm (table 3). Those that failed to be initiated on OAC had a mean and maximum CHA2DS2-VASc score of 4.6 (range 2–6; standard deviation [SD] 1.4) and 6, respectively. Those patients with a scheduled (n=25) versus non-scheduled (n=4) anticoagulant clinic appointment were commenced on OAC within a mean of six and 37 days, respectively.
Clinical events during mean follow-up of 194 days (range 76–365, table 3)
In the two patients (CHA2DS2-VASc score of 4) that suffered a stroke, OAC therapy was planned but was not started due to, either the GP not receiving the discharge summary, or failing to refer the patient for initiation of OAC therapy. There were no reported episodes of major bleeding among the patients who were commenced on OAC.
Discussion
Stroke is a potentially devastating condition, which is associated with rates of death and major disability of 7–10%11 and 10–12%,12 respectively. The efficacy of OAC in preventing AF-related strokes is well established. Despite this, only 82% of these patients in our cohort with CHA2DS2-VASc score ≥2, and no contraindications, were referred for OAC, and still fewer (69% of those in whom OAC was indicated) were initiated on such therapy.
These data add to the considerable weight of evidence, which shows that OAC is underused in patients with AF,11 irrespective of the way in which they present. Patients are only likely to receive OAC if their increased risk for stroke is recognised. In this study, 32 (14%) of 234 patients did not have a CHA2DS2-VASc score documented in their medical records. Among the 201 patients in whom OAC was clearly indicated, 22 (12%) patients did not undergo formal risk stratification for stroke. This emphasises the importance of education.
Among the patients who were considered for OAC, more than one-third were felt to have contraindications to therapy, with recurrent falls or gastrointestinal bleeding being most common, or the patient declined therapy. Many of the contraindications were relative ones, which do not preclude OAC if the stroke risk is high.13-15 A study has demonstrated that the calculated risk of subdural haematoma from falling was such that a patient with a 5% annual stroke risk for AF would need to fall 295 times in a year for the fall risk to outweigh the stroke reduction benefit of OAC.16 Among patients who are at increased risk of bleeding, clinicians appear more ready to prescribe aspirin than OAC therapy despite its limited efficacy for stroke prevention and a similar rate of major bleeding compared with OAC.8,17,18
Outpatient OAC therapy was associated with a high failure rate, and the main explanation for this, in more than 56% of cases, was poor discharge communication from secondary to primary care and administrative errors. Outpatient initiation of OAC therapy was also associated with considerable delays to initiation of treatment (mean 33 days). Patients who were dependent upon their GP to organise initiation of OAC therapy waited an average of three months before they were initiated on OAC therapy. By contrast, those who left hospital with a scheduled anticoagulation clinic appointment waited a significantly shorter time to start therapy (six days). It is difficult to see the rationale for introducing an additional step in the clinical pathway by asking the GP to organise OAC therapy. It is, thereby, the discharging team’s responsibility to organise outpatient anticoagulation. Interestingly, patients who had a CHA2DS2-VASc score above the mean were no more likely to be initiated on OAC as an inpatient than other patients (OR 0.72; 95% 0.39–1.34; p=0.31).
In our cohort, two patients that were intended to initiate OAC therapy but did not, suffered an ischaemic stroke. Inpatient OAC therapy initiation negates the possibility that it will not be started later, but there is a fear that the time to achieve therapeutic international normalised ratios (INRs) will prolong hospital stay. However, we have demonstrated that this approach does not cause longer hospital stays compared with outpatient initiation (median 6 vs. 7 days; p=0.42) and it has been reported that therapeutic INRs can be achieved within this timeline.19,20
Three NOACs are approved by the National Institute for Health and Care Excellence (NICE) for the prevention of non-valvular AF-related strokes.21 Despite faster onset of action compared with warfarin, in our centre, their use is restricted to those either allergic to warfarin or who have erratic INRs, and they are not widely prescribed. Indeed, in our cohort, only three patients were commenced on a NOAC. Their wider use might reduce the length of stay through obviating the need to achieve therapeutic INRs. A third approach, which was not used in this study, is to use a low molecular weight heparin in discharged patients until a therapeutic INR is achieved.
Limitations
The retrospective design of this study makes it more prone to bias than prospective studies. The composition of the study population, for example, was dependent upon the accuracy of hospital discharge coding data. Its accuracy for the positively identified cases was validated by a review of the medical records of a random subgroup of 80 patients among whom the discharge code I48 was correct in 79 (99%) cases. However, it is not known how many hospitalised patients with AF were not given the correct discharge code and were, consequently, not included in the study population.
In this study, we described physician-reported contraindications to OAC but we did not record whether bleeding risk was formally assessed using a tool such as the HAS-BLED score.
Conclusion
In this single-centre study of hospitalised patients with newly diagnosed AF, only 82% of patients with a CHA2DS2-VASc score ≥2 were referred for initiation of OAC, and still fewer actually received treatment. Outpatient referral for OAC therapy was associated with poor uptake and significant delays. Patient pathways that maximise treatment uptake and minimise delays are required.
Conflict of interest
None declared.
Editors’ note
See also the editorial in this issue.
Key messages
- Formal risk stratification for stroke is still not performed in all patients with atrial fibrillation (AF)
- A large group of patients with AF where oral anticoagulation (OAC) is indicated still fail to receive such therapy
- Inpatient OAC does not result in longer hospital stays
- Outpatient OAC is associated with poor uptake and significant delays
References
1. Chugh SS, Havmoeller R, Narayanan K et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–47. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.005119
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. http://dx.doi.org/10.1161/01.STR.22.8.983
3. Benn Christiansen C, Gislason G, Torp-Pedersen C et al. Primary prevention of ischemic stroke: implication of multiple risk factors in patients with and without atrial fibrillation. European Society of Cardiology 2013 Congress, Amsterdam, the Netherlands; 31 August 2013.
4. Marini C, De Santis F, Sacco S et al. Contribution of atrial fibrillation to incidence and outcome of ischaemic stroke: results from a population-based study. Stroke 2005;36:1115–19. http://dx.doi.org/10.1161/01.STR.0000166053.83476.4a
5. Lin HJ, Wolf PA, Kelly-Hayes M et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–4. http://dx.doi.org/10.1161/01.STR.27.10.1760
6. Jorgensen HS, Nakayama H, Reith J. Acute stroke with atrial fibrillation. The Copenhagen stroke study. Stroke 1996;27:1765–9. http://dx.doi.org/10.1161/01.STR.27.10.1765
7. Atrial Fibrillation Association. Why the UK must address the personal, clinical and economic impact of atrial fibrillation. June 2010. Available from: http://www.atrialfibrillation.org.uk/files/file/af-afa-keeping-your-finger-on-the-pulse-report.pdf
8. Andersen LV, Vestergaard P, Deichgraeber P et al. Warfarin for the prevention of systemic embolism in patients with non-valvular atrial fibrillation: a meta-analysis. Heart 2008;94:1607–13. http://dx.doi.org/10.1136/hrt.2007.135657
9. Capodanno D, Capranzano P, Giacchi G et al. Novel oral anticoagulants versus warfarin in non-valvular atrial fibrillation: a meta-analysis of 50, 578 patients. Int J Cardiol 2013;167:1237–41. http://dx.doi.org/10.1016/j.ijcard.2012.03.148
10. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Guidelines for the management of atrial fibrillation. Eur Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
11. Cowan C, Healicon R, Robson I et al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart 2013;99:1166–72. http://dx.doi.org/10.1136/heartjnl-2012-303472
12. Royal College of Physicians National Sentinel Stroke Clinical Audit 2010 Round 7. Public report for England, Wales and Northern Ireland, prepared on behalf of the Intercollegiate Stroke Working Party 2011. Available from: https://www.rcplondon.ac.uk/sites/default/files/national-sentinel-stroke-audit-2010-public-report-and-appendices_0.pdf
13. O’Brien EC, Holmes DN, Ansell JE et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J 2014;167:601–09. http://dx.doi.org/10.1016/j.ahj.2013.12.014
14. Rosenman MB, Simon TA, Teal E et al. Perceived or actual barriers to warfarin use in atrial fibrillation based on electronic medical records. Am J Ther 2012;19:330–7. http://dx.doi.org/10.1097/MJT.0b013e3182546840
15. Garwood CL, Corbett TL. Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother 2008;42:523–32. http://dx.doi.org/10.1345/aph.1K498
16. Man-Son-Hing M, Nichol G, Lau A et al. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677–85. http://dx.doi.org/10.1001/archinte.159.7.677
17. Assiri A, Al-Majzoub O, Kanaan AO et al. Mixed treatment comparison meta-analysis of aspirin, warfarin, and new anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation. Clin Ther 2013;35:967–84. http://dx.doi.org/10.1016/j.clinthera.2013.05.011
18. Mant J, Hobbs FD, Fletcher K et al. BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493–503. http://dx.doi.org/10.1016/S0140-6736(07)61233-1
19. Crowther MA, Ginsberg JB, Kearon C et al. A randomized trial comparing 5mg and 10mg warfarin loading disease. Arch Intern Med 1999;159:46–8. http://dx.doi.org/10.1001/archinte.159.1.46
20. Harrison L, Johnston M, Massicotte MP, Crowther M, Moffat K, Hirsh I. Comparison of 5mg and 10mg loading doses in initiation of warfarin therapy. Ann Intern Med 1997;126:133–6. http://dx.doi.org/10.7326/0003-4819-126-2-199701150-00006
21. NICE Implementation Collaborative. Consensus. Supporting local implementation of NICE guidance on use of the novel (non-Vitamin K antagonist) oral anticoagulants in non-valvular atrial fibrillation. London: NICE, June 2014. Available from: https://www.nice.org.uk/guidance/cg180/resources/cg180-atrial-fibrillation-nic-consensus-statement-on-the-use-of-noacs2