People with chronic heart failure (CHF) often experience exacerbations of their symptoms that require hospitalisation. The feasibility, safety and efficacy of early post-exacerbation rehabilitation are largely unknown in this population.
This was a single-centre, feasibility trial of early rehabilitation versus usual care. Those assigned to the intervention started rehabilitation within 10 days of discharge and attended supervised sessions twice per week for eight weeks. The primary outcomes were feasibility of uptake and safety. Assessments were performed at baseline and three months: exercise tolerance (shuttle walking tests) and health status (questionnaire).
There were 1,298 patients screened, 16 patients recruited (<1% of those screened) and 11 randomised (five rehabilitation, six control). The primary reasons for exclusion were contraindication to exercise and normal ejection fraction. There were improvements in exercise tolerance and health status in both groups at three months; however, the study was not powered to report any within- or between-group significance. The early rehabilitation intervention was safe with no adverse events reported.
In conclusion, early rehabilitation, for patients with CHF, was unfeasible. The 10-day recruitment target was too restrictive in this population. This is important because there has been a drive towards early rehabilitation in CHF guidelines.
Introduction
People with chronic heart failure (CHF) often experience acute exacerbations (AE) of their symptoms,1,2 which frequently require a hospital admission. This poses a burden on the individual and the health service.2
A number of systematic reviews have now confirmed the benefits of exercise training in those with CHF.3,4 There is strong evidence to recommend exercise training to all stable heart failure patients,1 and relatively few complications during training have been observed.5 However, the role of exercise immediately after an AE of CHF has not been established, although there is compelling evidence in chronic obstructive pulmonary disease (COPD).6 Despite a paucity of evidence in this area, there has been a drive towards providing early post-hospitalisation (within 10 days of discharge) exercise within several important guidelines for the management of patients with heart disease.7-9
We have previously reported the pilot data in support of the current study.10 Results showed that rehabilitation was effective in terms of improving exercise tolerance and reduced depression. No adverse events were reported. However, there was no control group and, therefore, the effects of natural recovery after illness were unaccounted for.
Objective
To conduct a feasibility trial of an early rehabilitation intervention for patients admitted with an AE of CHF compared with usual care.
Materials and methods
Patients admitted to the acute medical wards of the trial hospital (Glenfield, Leicester, UK) were invited to participate in the study. All participants gave written informed consent and the study was approved by Leicestershire, Northamptonshire & Rutland Ethics Committee (Reference: 10/H0402/48).
Inclusion criteria
Patients were included if they had a diagnosis of CHF confirmed by left ventricular systolic dysfunction (LVSD) on echocardiogram and had impaired exercise tolerance (New York Heart Association [NYHA] classifications II–IV).11
Exclusion criteria
Usual exclusion criteria to our current outpatient exercise programme applied (these criteria reflect international guidelines).12 Patients were furthermore excluded if they had terminal disease, had four or more admissions within the last year or if they had attended rehabilitation in the preceding 12 months.
Study design
This was a prospective, single-blind, feasibility, randomised-controlled trial (RCT) comparing early rehabilitation with usual care. Assessments were carried out up to 24 hours prior to hospital discharge (or within 48 hours of discharge) and at three months. Assessments were performed by a blinded assessor.
Usual care
Patients in the control group were discharged with ‘best usual’ care. This involved: follow-up in primary care by the heart failure specialist nursing (HFSN) team, an outpatient appointment with a cardiologist and were offered rehabilitation after three months.
Intervention
Those assigned to the intervention group received ‘best usual’ care in addition to the early rehabilitation programme. The rehabilitation consisted of supervised exercise training and self-management education, delivered by a multi-disciplinary team, which started within 10 days of hospital discharge. Patients attended two classes per week for eight weeks. Patients were closely monitored for symptoms (Borg scores,13 heart rate, and blood pressure).
Outcome measures
- Feasibility: study uptake.
- Safety, tolerability and acceptability: any adverse events were recorded for the duration of the study, e.g. infection, deterioration, admission to hospital. To determine the safety and tolerability of the intervention for individual patients, recording any adverse responses to exercise also occurred. Adherence to the rehabilitation intervention and reasons for attrition were also recorded.
- Functional exercise capacity: walking performance was measured at hospital discharge and three months using the incremental shuttle walk test (ISWT)14 (metres) and the endurance shuttle walking test (ESWT)15 (seconds).
- Quality of life: at hospital discharge and three months, health status was captured using the Minnesota Living With Heart Failure Questionnaire (MLWHFQ).16
Results
Screening
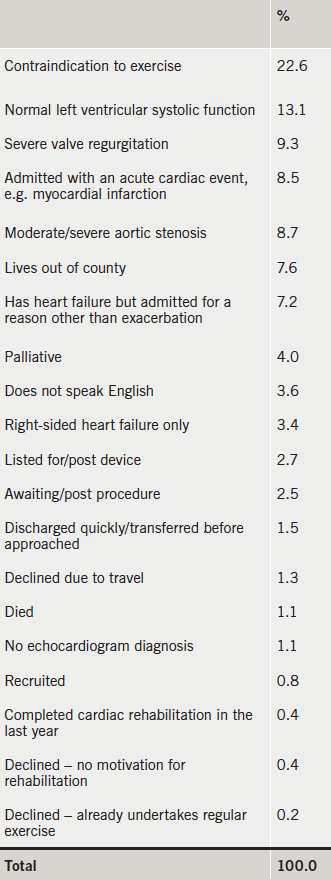
Screening took place from November 2010 to June 2012. The total number of patients screened was 1,298. This was approximately 68 per month or 17 per week (taking into account holiday periods). The total number of patients recruited were 16 (<1% of those screened). Table 1 highlights the reasons for exclusion/non-uptake.
Baseline characteristics
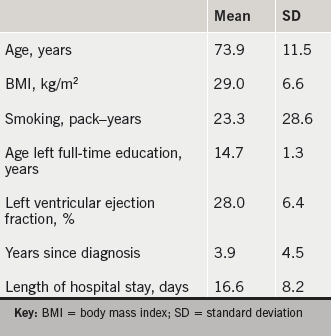
Table 2 outlines the baseline characteristics of recruited participants. Ten subjects were male, nine had concurrent right-sided heart failure. The primary causes for heart failure were coronary artery disease and previous myocardial infarction. All were NYHA grades III–IV.
Participant flow
Figure 1 outlines the participant flow through the study and reasons for attrition in both groups.
Outcome measures
No deaths occurred during the study period. Two patients in the rehabilitation group had a single hospitalisation (for a cardiac cause – further exacerbation leading to attrition). These episodes lasted for nine days for one subject and two days for the other. There were no hospitalisations in the control group but a single Accident and Emergency (A&E) visit for a non-cardiac cause. Patients in the rehabilitation group attended an average of 8.4 rehabilitation sessions (standard deviation [SD] 7.1). Two patients attended all 16 sessions. There were no adverse events during the exercise sessions. Improvements were observed in both groups, of similar magnitude for the ISWT (intervention: mean change 80.0, 95% confidence interval [CI] –242.9–402.9 m; control: mean change 68.3, 95% CI –13.3–150.0 m), the ESWT (intervention: mean change 434.0, 95% CI –952.8–1820.8 s; control: mean change 365.7, 95% CI –159.3–890.6 s) and the MLWHFQ (intervention: mean change –19.3, 95% CI –61.6–23.0; control: mean change –19.6, 95% CI –37.0–2.2).

Discussion
We found that the provision of early rehabilitation, after an AE was unfeasible in this population. This finding is important; particularly given the drive towards early rehabilitation in several guidelines,7-9 and for other important long-term conditions.6 The number of patients screened compared with the number of patients recruited was very low. This study was not powered to compare within- or between-group significance for other outcomes, such as exercise tolerance and quality of life, however, the control group did show signs of natural recovery, again observed in a recent UK trial in COPD.17
The impracticality of this approach was surprising. It may be that some factors have changed since our pilot work10 was carried out. First, we now have an expert HFSN team. They are able to manage AE of CHF in the community, in less severe patients. Therefore, only those with complex needs and comorbidities are admitted to hospital. These patients are often not suitable to engage in exercise, and this was, by far, the most prevalent exclusion criteria, accounting for 22.6%. In addition, our pilot work10 had a four-week window to start people on the rehabilitation programme. This suggests that the 10-day target imposed in this study was too challenging in this patient group.
The second largest reason for exclusion to the study was normal left ventricular systolic (LVS) function, despite a clinical diagnosis of CHF. This group have been termed HFNEF (heart failure with normal ejection fraction) in the literature.4 The pathophysiology behind exercise intolerance in HFNEF is not well understood, and we, therefore, excluded these patients from our sample. However, there is some evidence to support rehabilitation in this group,18 and this may be a future research priority.
Despite poor recruitment to the study, in those patients attending rehabilitation, the intervention was safe (no adverse events). In conclusion, early rehabilitation, following an AE, is not feasible in the CHF population.
Key messages
- Early rehabilitation, delivered within 10 days of hospital discharge, in patients with chronic heart failure is safe
- However, early rehabilitation, is unfeasible in this setting
- The 10-day recruitment target was too restrictive in this population
- The primary reasons for exclusion were contraindication to exercise and a normal ejection fraction
Acknowledgements and support
The research was funded by the National Institute for Health Research (NIHR) Leicestershire Northamptonshire & Rutland (LNR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) (now CLAHRC-East Midlands [EM]), and took place at the University Hospitals of Leicester NHS Trust. Support was also provided by the NIHR Leicester Respiratory Biomedical Research Unit (BRU). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Trial Registration: ISRCTN56811468.
Conflict of interest
None declared.
References
1. Dickstein K, Cohen-Solal A, Filippatos G et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–442. http://dx.doi.org/10.1093/eurheartj/ehn309
2. Cleland JG. Contemporary management of heart failure in clinical practice. Heart 2002;88(suppl 2):ii5–ii8. http://dx.doi.org/10.1136/heart.88.suppl_2.ii5
3. Davies EJ, Moxham T, Rees K et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2010;(4):CD003331. http://dx.doi.org/10.1002/14651858.cd003331.pub3
4. Taylor RS, Sagar VA, Davies EJ et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;(4):CD003331. http://dx.doi.org/10.1002/14651858.CD003331.pub4
5. O’Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. http://dx.doi.org/10.1001/jama.2009.454
6. Puhan MA, Spaar A, Frey M et al. Early versus late pulmonary rehabilitation in chronic obstructive pulmonary disease patients with acute exacerbations: a randomized trial. Respiration 2011;83:499–506. http://dx.doi.org/10.1159/000329884
7. British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and rehabilitation. Second edition. London: BACPR, 2012. Available from: http://www.bacpr.com/resources/46c_bacpr_standards_and_core_components_2012.pdf
8. National Institute for Health and Care Excellence. CG108. Chronic heart failure: management of chronic heart failure in adults in primary and secondary care guidelines. London: NICE, 2010. Available from: https://www.nice.org.uk/guidance/cg108
9. Piepoli MF, Conraads V, Corrà U et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347–57. http://dx.doi.org/10.1093/eurjhf/hfr017
10. Houchen L, Watt A, Boyce S, Singh S. A pilot study to explore the effectiveness of “early” rehabilitation after a hospital admission for chronic heart failure. Physiother Theory Pract 2012;28:355–8. http://dx.doi.org/10.3109/09593985.2011.621015
11. Scrutinio D, Lagioia R, Ricci A, Clemente M, Boni L, Rizzon P. Prediction of mortality in mild to moderately symptomatic patients with left ventricular dysfunction. The role of the New York Heart Association classification, cardiopulmonary exercise testing, two-dimensional echocardiography and Holter monitoring. Eur Heart J 1994;15:1089–95. Available from: http://eurheartj.oxfordjournals.org/content/15/8/1089
12. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 8th edn. Baltimore: Lippincott Williams and Wilkins, 2009.
13. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. http://dx.doi.org/10.1249/00005768-198205000-00012
14. Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–24. http://dx.doi.org/10.1136/thx.47.12.1019
15. Revill SM, Morgan MD, Singh SJ, Williams J, Hardman AE. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax 1999;54:213–22. http://dx.doi.org/10.1136/thx.54.3.213
16. Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 2: Content, reliability and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Failure 1987;3:198–209.
17. Greening NJ, Williams JEA, Hussain SF et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315. http://dx.doi.org/10.1136/bmj.g4315
18. Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 2010;3:659–67. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.110.958785
