Enhanced understanding of organ fibrosis and novel therapeutic targets were the focus of many presentations at last year’s 11th Annual Scientific Meeting of the Cardiorenal Forum, held at the Royal College of Obstetricians and Gynaecologists, London, on 6th October 2016. Dr Richard Crawley reports on its highlights.
Targeting uric acid

The conference’s keynote lecture, delivered by Professor Austin Stack (University Hospital, Limerick, Ireland), homed in on the idea that serum uric acid directly contributes to increased cardiovascular disease. This was shown in his team’s work published in 2013,1 which used retrospective data to identify a direct correlation between raised serum uric acid concentrations and increased risk of developing cardiovascular disease.
This, therefore, begs two questions:
- Firstly, does uric acid directly cause vascular endothelial damage, contributing to acute renal dysfunction – thereby increasing the risk of cardiovascular disease?
- Secondly, does uric acid increase the risk of hypertension, chronic kidney disease and diabetes mellitus – which, in turn, increases the risk of cardiovascular problems?
An increase in serum uric acid is thought to result in a proportional fall in nitric oxide levels by decreasing its production. Certainly, animal studies artificially inducing hyperuricaemia have resulted in endothelial damage, and this is separate to the damage caused by reactive oxygen species (ROS) produced by xanthine oxidase.2
In humans, there have been several studies to suggest that in those with major cardiovascular co-morbidities such as hypertension, type 2 diabetes and chronic kidney disease, higher levels of serum uric acid have also been found. This previously was thought to be a result of renovascular damage and nephrosclerosis,3 but more recent studies have shown hyperuricaemia to be a predictor of onset. This risk of comorbidity has been seen to be significantly higher in the higher quartiles of uric acid concentration, suggesting a further direct correlation.
What then can be done? Is there a role for xanthine oxidase inhibitors such as allopurinol? Certainly, evidence from a study looking at adolescent hypertension suggested that taking allopurinol for only four weeks was enough to significantly reduce blood pressure in two thirds of the participants.4 Despite the encouraging results, this small study had many limitations. Further studies in humans have suggested that allopurinol use slows deterioration in renal function, and that contributes to a reduced risk of cardiovascular events.5 Professor Stack highlighted though that no large randomised control trials have yet demonstrated the beneficial effects of allopurinol in reducing cardiovascular risk, and perhaps it is time for us to focus on uric acid – a forgotten risk factor.
SGLT-2 inhibitors: a game changer?
The results of the long awaited EMPA-REG (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) trial were discussed by Dr Amar Puttanna (Young Diabetologists and Endocrinologists Forum, and West Midlands Deanery), then later mentioned in both the cardiology and renal trials updates, by Professor John Cleland (Imperial College, London) and Dr Patrick Mark (Queen Elizabeth University Hospital, Glasgow), respectively.
SGLT-2 inhibitors, such as empagliflozin, are thought to reduce the level of glucose reabsorption at the nephron, resulting in higher urinary glucose concentrations.6 However, no direct link has previously been shown between lowering serum glucose levels and cardiovascular risk. EMPA-REG has sought to investigate this issue, and the implications of this trial are certainly exciting for those working in both diabetes and cardiology.
Evidence published last year suggests an overwhelming benefit of empagliflozin in preventing cardiovascular disease in comparison to placebo.7 Although there was no difference in the incidence of stroke or myocardial infarction, there was evidence suggesting significantly lower rates of death from cardiovascular causes and lower rates of hospitalisation for symptoms associated with heart failure. Interestingly, the large population recruited already had evidence of cardiovascular disease, and a relative risk reduction of 38% for further cardiovascular events in such a high risk population is impressive.
Another major finding from this trial is the safety of empagliflozin in the long term. There were slight increased risks of genital and urinary tract infections, but no obvious deterioration in renal function. Given the safety and efficacy in reducing serum glucose and HbA1c levels, even in those with CKD Stage II & III,8 it is likely that SGLT-2 inhibitors would be particularly useful in patients with multiple co-morbidities, where other more diabetic medications come into trouble.
What’s more, there is increasing evidence from EMPA-REG to suggest that empagliflozin has a diuretic effect from increased glucose excretion, and this may be the reason for the improved outcomes in chronic heart failure patients, particularly lower rates of hospitalisation from symptoms.
Slowing fibrosis

Professor Tim Johnson (University of Sheffield, and UCB New Medicines) discussed the implication of fibrosis (see table 1) in organ scarring in the Western world, indicating that it is involved in 45% of all organ death. Our targeting for future therapeutics, however, hasn’t been particularly successful of yet.
One example is tissue growth factor (TGF-β), which is a powerful regulator of fibrotic disease throughout the body. Despite the money spent by drug companies to investigate this, the autoimmune side effects have been too widespread. Similarly, peroxisome proliferator-activated receptor (PPAR) agonists are used in diabetes and hyperlipidaemia, but their adverse effects profiles – particularly fluid retention and decompensation of heart failure symptoms – have prevented use as an antifibrotic drug in patients.
More promisingly, lysophosphatidic acid receptors (LPAR) are associated with cell proliferation and maintenance throughout the body. This is thought to be associated with increased intracellular calcium mobilisation. The signalling pathway associated with this is thought to directly contribute to several diseases and, as such, several receptor antagonists are currently in phase II production. They have shown promising results with regards to fibrosis and, along with autotaxin inhibition, may become available for use in the coming years.9 It is likely, though, that we will need to consider multi-drug therapies if we are to slow organ death associated with fibrosis.
Novel functional imaging techniques
An interesting talk regarding new imaging techniques based around magnetic resonance (MR), was led by Dr Aghogho Odudu (University of Manchester). The use of MR in cardiac and renal imaging is clearly growing, and the use of gadolinium to enhance both blood and extracellular fluid is a testament to the effectiveness of functional MR imaging.
Blood-oxygen-level dependent imaging (BOLD) works off of the basis that both oxygenated and deoxygenated haemoglobin are both weakly magnetic. The spin of both molecules can alter the signal and this has been useful in renal imaging – particularly identifying the loss of capillary beds in renal artery stenosis. Arterial spin labelling takes this further with real-time perfusion images – for example, demonstrating differences in the perfusion of both renal cortex and medulla. This has the potential to demonstrate both areas damaged by acute tubular injury. From a cardiac perspective, it also has the potential to be an extremely sensitive functional assessment of perfusion damage in myocardial ischaemia.
In non-ischaemic cardiomyopathies, it is thought that myocardial fibrosis is the primary underlying cause. This change in extracellular matrix remodelling increases the risk of diastolic heart failure and arrhythmias.10 Areas of specific regional myocardial scarring seen in cardiac ischaemia have a far slower washout time with regards to gadolinium-enhancement and, as such, are seen as an increased signal intensity. Diffuse fibrosis is more difficult to establish, and post-contrast T1 cardiac MR mapping has a role in identifying this. Consequently, T1 mapping is far more effective in detecting pathologies, such as dilated cardiomyopathy.11 This technique may soon be used also in renal imaging – in particular for characterising the scarring seen in (CKD) kidney disease and end-stage renal failure.
The PCSK9 inhibitor phenomenon
The work of Professor Jennifer Robinson (University of Iowa, USA) and her colleagues as part of the ODYSSEY trial has been pivotal in demonstrating the effectiveness of the PCSK9 inhibitor, alirocumab. Her lecture was timely given the recent National Institute of Health and Care Excellence (NICE) recommendations (TA393) regarding the use of alirocumab in primary hypercholesterolaemia and mixed dyslipidaemia (see table 2).12
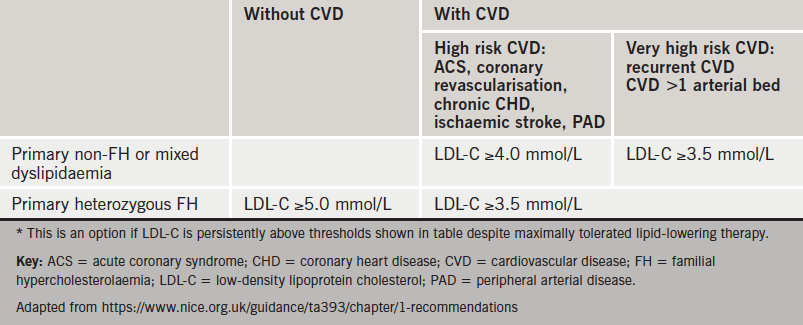
The efficacy of this new drug is without question. The ODYSSEY trial13 showed that when used in conjunction with statin therapy, alirocumab demonstrated a 62% drop in calculated serum low-density lipoprotein (LDL) within the first few weeks when compared to placebo.
At 78 weeks, the reduction in calculated serum LDL was still 52% lower than placebo plus statin. Alongside this, the team also demonstrated a significant reduction in cardiovascular risk, with the total number of adverse events related to cardiovascular causes just over half those seen in the placebo group (1.7% vs. 3.3%).
Although the benefits of alirocumab with concomitant statin use are there for all to see, the side effects profile remains a point of contention. The degree of myalgia was double that of the placebo plus statin group, and there have been question marks raised regarding the neurological safety of the drug – several episodes of amnesia and ophthalmological events were seen in the alirocumab treatment arm during the ODYSSEY trial. Although these were not deemed significant in the study, long-term safety remains an ongoing concern. Results from further trials are due to follow in 2017/18, with closer attention to the adverse effects and safety profile of what could be a very exciting drug for dyslipidaemia.
Atrial fibrillation and CKD
CKD, however we measure it, “signifies poor outcomes in atrial fibrillation patients”. This was a key message from Dr Ami Banerjee (University College London). Drawing on recent European Society of Cardiology guidelines,14 he outlined how atrial fibrillation (AF) is present in 15–20% of CKD patients (10–20 times higher than the general population). These patients have an increased stroke rate, and there is also a higher risk of bleeding in CKD. The big question in terms of non-valvular AF and kidney disease is: how are they related?
Guidelines suggest that renal function should be assessed in anyone with new AF, particularly in those with bleeding episodes, when considering oral anticoagulation (OAC). Impairment in renal function is included as part of several bleeding risk scores (e.g.HAS-BLED) and anticoagulation may be ruled out in those with high scores. Whilst monitoring AF patients on OACs, renal function should be checked regularly, and whilst safe in moderate and moderate-to-severe CKD (eGFR >15), dose adjustment should be considered for those on non-vitamin K novel oral anticoagulation (NOACs).
Around 20% of patients in trials with the four NOACS had renal dysfunction, but no studies included patients with end-stage renal disease. It also appears that in clinical practice there is reluctance throughout Europe to give renally impaired patients the full drug dose, and that a lower dose is given even in patients with mild kidney impairment. Despite that, data from The Health Improvement Network (THIN) database, a national GP prescribers’ database, showed that in the UK there is greater prescribing of NOACs in moderate and moderate to severe renal failure than warfarin.
A recently published meta-analysis of four randomised controlled trials (RCTs) compared efficacy and safety (major bleeding) of NOACs versus warfarin in over 58,000 patients, including data on renal function.15 It showed that use of NOACs was associated with a reduced risk of stroke, systemic embolism and major bleeding compared to warfarin in individuals with mild or moderate renal impairment, suggesting a favourable risk profile for these agents in patients with renal disease. However, there is very little data regarding the use of NOACs in end-stage renal disease, and the severe end of the spectrum appears to be neglected. At this time, there are no RCTs assessing OACs in haemodialysis patients or following kidney transplantation, nor studies of NOACs in those with severe CKD (i.e. a creatinine clearance <25–30ml/min). This severe end of the CKD spectrum needs to be addressed in the future.
IV iron in heart failure
The final lecture of the day, given by Professor Philip Kalra (University of Manchester), helped to elaborate on one of the hot topics in heart failure – the ongoing IRONMAN study.
This has stemmed from work done approximately 15 years ago demonstrating that the use of intravenous (IV) iron (and subcutaneous erythropoietin) resulted in both an improvement in cardiac function (left ventricular ejection fraction)16 and a decrease in hospitalisation associated with heart failure symptoms.17 Although IV iron fell out of fashion, this area is being studied again via the large multi-centre trial. IRONMAN is looking at both long-term cardiovascular mortality and frequency of hospital admissions for heart failure in those with sufficiently low serum iron and transferrin saturations.
The adverse effects from IV iron have been widely debated and patients should have the correct education prior to being recruited to the study. At present, short-term adverse effects include reactions to the infusion – either hypersensitivity or labile reactions. The IV iron in use is iron isomaltoside (Monofer™) and whilst, in the past, ‘test doses’ to IV iron were needed, they are currently not indicated due to the relative safety of this particular preparation. In the longer term, there has been discussion about IV iron increasing the risk of infection if used in chronic disease. Recent studies have now suggested that this is not correlated but it is important to remember that IV iron should not be given during active infection.
Furthermore, the intended benefits from IRONMAN may be enhanced further following the increasing use of sacubitril/valsartan. This was approved for use in April 2016 by NICE (TA388),18 following the overwhelming evidence that it showed improved outcomes in severe symptomatic heart failure when compared with enalapril (PARADIGM-HF trial19). The crossover between the two treatments is unlikely to have a significant bearing on the outcomes of IRONMAN but certainly this is an exciting time for therapeutics associated with heart failure.
Richard Crawley
Cardiology Registrar
Portsmouth Hospitals NHS Trust
Acknowledgment
The Cardiorenal Forum would like to thank the following companies for their generous support of the 2016 meeting: A Menarini Farmaceutica Internazionale S.R.L., Bristol-Myers Squibb, MSD, Novartis, Pfizer, Pharmacosmos, Sanofi, Servier, Shire, UCB, Vifor Fresenius Medical Care Renal Pharma.
References
1. Stack AG, Hanley A, Casserly LF, et al. Independent and conjoint associations with gout and hyperuricaemia with total and cardiovascular mortality. Q J Med 2013;106:647–58. http://doi.org/10.1093/qjmed/hct083
2. Khosla UM, Zharikov S, Finch JL, et al. Hyperuricaemic induces endothelial dysfunction. Kidney Int 2005;67:1739–742. http://doi.org/10.1111/j.1523-1755.2005.00273.x
3. Messerli FH, Frohlich ED, Dreslinski GR, et al. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med 1980;93:817–21.
4. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 2008;300:924–32. http://doi.org/10.1001/jama.300.8.924
5. Goicoechea M, García de Vinuesa S, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. CJASN 2010;5:1388–93. http://doi.org/10.2215/CJN.01580210
6. Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015;12:78–89. http://doi.org/10.1177/1479164114561992
7. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. http://doi.org/10.1056/NEJMoa1504720
8. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. LanDia 2014;2:369–84. http://doi.org/10.1016/S2213-8587(13)70208-0
9. Stoddard NC, Chun J. Promising pharmacological directions in the world of lysophosphatidic acid signaling. Biomol Ther 2015;23:1–11. http://doi.org/10.4062/biomolther.2014.109
10. Ling LH, Kistler PM, Ellims AH, et al. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol 2012;60: 2402–08. http://doi.org/10.1016/j.jacc.2012.07.065
11. Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 2008;52:1574–80. http://doi.org/10.1016/j.jacc.2008.06.049
12. National Institute for Health and Care Excellence. Technology Appraisal Guidance (TA393): alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. NICE: June 2016. https://www.nice.org.uk/search?q=TA393
13. Robinson J, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–99. http://doi.org/10.1056/NEJMoa1501031
14. Kirchhof P, Benussi S, Kotecha D, et al. (The Task force for the Management of Atrial Fibrillation of the European Society of Cardiology). ESC 2016 European Society of Cardiology Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;117:1–90. http://doi.org/10.1093/ejcts/ezw313
15. Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol 2016;17:69–75. https://doi.org/10.1016/j.amjcard.2015.09.046
16. Silverberg DS, Wexler D, Sheps D et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol 2001;37:1775–80. https://doi.org/10.1016/S0735-1097(01)01248-7
17. Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 2000;35:1737–44.
18. National Institute for Health and Care Excellence. Technology Appraisal Guidance (TA388): Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. NICE: April 2016. https://www.nice.org.uk/search?q=TA39
19. McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus Eenalapril in heart failure (PARADIGM-HF). N Engl J Med 2014;371: 993–1004. http://doi.org/10.1056/NEJMoa1409077
