Background
Thrombus formation in the coronary tree is the principal cause of acute coronary syndromes (ACS).1 Following plaque rupture or erosion, platelets adhere to exposed ligands (collagen, von Willebrand factor [vWF]) under high-flow conditions and this leads to platelet activation. Following platelet adhesion and activation, multiple agonists are secreted, including thromboxane A2 (TXA2) and adenosine diphosphate (ADP). TXA2 further activates platelets and ADP amplifies and sustains platelets’ activation, particularly through platelet P2Y12 receptors.2 In view of the pivotal role of platelets in arterial thrombosis, blocking TXA2 production with aspirin and platelet P2Y12 receptors with a P2Y12-receptor antagonist has been successful in reducing major cardiovascular events (MACE) following ACS. Until recently, dual antiplatelet therapy (DAPT) with aspirin and clopidogrel for 12 months following ACS was the standard of care. However, limitations with clopidogrel, including a high rate of ‘non-responders’ and relatively slow and variable onset/offset of action, have led to the development of the more potent P2Y12 inhibitors, prasugrel and ticagrelor.3
The risk following ACS remains high beyond the initial 12-month period. Among those who do not have a major event in the first year, approximately 20% will die or suffer a non-fatal myocardial infarction (MI) or stroke within the following three years.4 Long-term DAPT has been successful in the PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) trial5 and the DAPT study6 in reducing ischaemic events, with a penalty of increased non-fatal bleeding events. It is, therefore, becoming clear that treatment should be individualised and the ‘one-size-fits-all’ approach is no longer acceptable. In this review, we will explore the evidence for different oral P2Y12 inhibitors for 12-months post-ACS and beyond.
12-month DAPT
Clopidogrel
Clopidogrel is a prodrug that belongs to the thienopyridine family. Its active metabolite irreversibly inhibits platelet P2Y12 receptors. Clopidogrel metabolism is complex, requiring conversion to the active metabolite by the cytochrome P450 (CYP) enzymes in the liver, and the majority (85%) of clopidogrel is catabolised to inactive metabolites by plasma carboxylesterases.7 This is largely responsible for the wide inter-individual variability in platelet reactivity during treatment with clopidogrel and ‘poor response’ to clopidogrel has been shown to increase ischaemic risk.8
In non-ST-elevation ACS (NSTE-ACS), clopidogrel, in addition to aspirin, was tested in the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) study. Clopidogrel, for three to 12 months post-NSTE-ACS, reduced MACE compared with placebo (9.3% vs. 11.4%, p<0.001). Despite a trend towards better cardiovascular mortality with clopidogrel, the MACE reduction was primarily driven by a reduction in non-fatal MI. There was no significant difference in terms of life-threatening or intracranial bleeds but clopidogrel resulted in a significant increase in major bleeding events (3.7% vs. 2.7%, p=0.001).9 Patients received clopidogrel at randomisation (within 24 hours of presentation, pre-percutaneous coronary intervention [PCI]) and those requiring PCI in the placebo arm received open-label clopidogrel or ticlopidine for ~four weeks post-PCI. Patients pre-treated with clopidogrel had a 31% reduction in the risk of MI or cardiovascular death (p=0.002).10
Clopidogrel is the only P2Y12 inhibitor tested in conjunction with aspirin and thrombolysis in ST-elevation ACS (STE-ACS) patients. The addition of clopidogrel offered a highly significant 41% reduction in occlusion of the infarct-related artery.11 In medically-treated STE-ACS patients, clopidogrel, in addition to aspirin, reduced MACE by 9% (p=0.002).12
Prasugrel
Prasugrel is another thienopyridine prodrug. Its active metabolite is chemically similar to that of clopidogrel and also inhibits the P2Y12 receptor irreversibly. It provides more rapid and potent platelet inhibition as compared with clopidogrel due to more efficient active metabolite formation, starting with intestinal esterase followed by a one-step conversion in the liver by multiple CYP enzymes.13 Prasugrel, compared with clopidogrel, reduced MACE (9.9% vs. 12.1%, p<0.001) in PCI-treated ACS patients in the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel – Thrombolysis In Myocardial Infarction 38) trial,14 an outcome that was predominantly driven by a reduction in MI (7.4% vs. 9.7%, p<0.001). Patients with NSTE-ACS were randomised only if PCI was indicated following coronary angiography. STE-ACS patients were included either following initial medical management and subsequent coronary angiography or if they were planned to receive primary PCI. Patients with diabetes and STE-ACS had the biggest reduction in ischaemic events. The downside was an increase in Thrombolysis In Myocardial Infarction (TIMI)-defined major bleeding, including life-threatening bleeds, in the prasugrel-treated group (2.4% vs. 1.8%, p=0.03). There was also a marginal but significant increase in fatal bleeding with prasugrel (0.4% vs. 0.1%, p=0.002). Despite this, subgroup analyses suggested net clinical benefit, except for those with history of stroke, low body weight (<60 kg) or age >75 years.14
The superior efficacy of prasugrel compared with clopidogrel was not replicated in medically-treated ACS patients in the TRILOGY (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) trial.15 Interestingly, major bleeding was similar in both clopidogrel- and prasugrel-treated patients. This difference in bleeding profile compared with the previous trial might be explained by a reduction in prasugrel dose in patients with low body weight (<60 kg) and those >75 years old.15
The ACCOAST (Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction) trial compared prasugrel pre-treatment in NSTE-ACS patients to treatment post-coronary angiography only for those undergoing PCI and found no difference in ischaemic end points but significantly higher rates of major bleeding (2.6% vs. 1.4%, p=0.006).16 Although this trial had limitations (over 50% of procedures were performed through the femoral approach, the study protocol did not recommend a wash-out period prior to coronary artery bypass surgery [CABG] and the majority of bleeding events were related to access site and surgery), this has put an end to prasugrel treatment in NSTE-ACS patients prior to coronary angiography.17
Ticagrelor
Ticagrelor belongs to a different class (cyclopentyl-triazolopyrimidines). It acts directly and reversibly blocks platelet P2Y12 receptors in a non-competitive pattern (allosteric inhibition). Being an active drug, ticagrelor achieves rapid platelet inhibition and minimises any chance of inter-individual variance in pharmacodynamic response.18 Ticagrelor is metabolised via hepatic CYP3A and, consequently, concomitant use of strong CYP3A inducers or inhibitors is contraindicated.19 Ticagrelor also has a more rapid and consistent offset of action compared with clopidogrel – a desired effect should serious bleeding occur or if major surgery is planned.18
Ticagrelor (90 mg twice daily) reduced MACE in moderate- to high-risk ACS patients compared with clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial (9.8% vs. 11.7%, p<0.001).20 This was driven by a reduction in MI (5.8% vs. 6.9%, p=0.005) and cardiovascular death (4% vs. 5.1%, p=0.001). Death from any cause was also reduced in ticagrelor-treated patients (4.5% vs. 5.9%, p<0.001). Although overall major, fatal and life-threatening bleeding rates were similar in both groups, non-CABG major bleeding was more with ticagrelor (4.5% vs. 3.8%, p=0.03). Intracranial haemorrhage was also marginally increased with ticagrelor (0.3% vs. 0.2%, p=0.06). Other important adverse effects of ticagrelor included dyspnoea (13.7% vs. 8.7%, p<0.001) and more frequent ventricular pauses in the first week of treatment (5.8% vs. 3.6%, p=0.01). Despite this, there was no increase in syncope or pacemaker implantation and the majority of dyspnoea events were mild or moderate with a minority having to discontinue treatment.21 More reassuringly, ticagrelor treatment did not have negative effects on cardiac or pulmonary function.22,23 Subgroup analyses confirmed consistent benefit with ticagrelor, including patients with STE-ACS,24 diabetes mellitus (DM)25 or chronic kidney disease (CKD)26 and the elderly.27 The benefit of ticagrelor was attenuated in patients recruited in the USA. Although this might have been due to chance (the USA cohort was only ~8% of the PLATO patients), the majority of patients in the USA were receiving a higher maintenance dose of aspirin (>300 mg daily).28 This raised the hypothesis of a negative interaction between higher doses of aspirin and ticagrelor, which remains to be further explored.
Ticagrelor also blocks cellular uptake of adenosine by erythrocytes and other cells through inhibition of the equilibrative nucleoside transporter 1 (ENT-1).29,30 It remains uncertain whether this explains some of ticagrelor’s beneficial effects, as well as some of its side effects.31
DAPT beyond 12 months
The PEGASUS-TIMI 54 trial5 assessed the efficacy and safety of ticagrelor 60 mg or 90 mg twice daily in patients with MI one to three years previously. There were 21,162 patients randomised to placebo, ticagrelor 60 mg or ticagrelor 90 mg. All patients received aspirin (75–150 mg daily). Patients were at least 50 years of age, with one additional marker of increased risk (age >65 years, DM requiring treatment, an additional previous MI, multi-vessel coronary artery disease or CKD not requiring dialysis). Patients with stroke (ischaemic or haemorrhagic), central nervous system tumour, or intracranial vascular abnormality, or those treated with anticoagulants, cilostazol or dipyridamole were excluded. The trial also excluded patients with gastrointestinal bleed within six months and those who had undergone major surgery within four weeks. Both the 60 mg and 90 mg twice-daily doses of ticagrelor reduced three-year MACE rates compared with placebo (7.77% vs. 9.04%, p=0.004 and 7.85% vs. 9.04%, p=0.008, respectively). Pooled analysis of both ticagrelor groups showed a non-significant trend towards improved cardiovascular mortality with ticagrelor compared with placebo (2.9% vs. 3.39%, p=0.06). There was no increase in rates of fatal or intracranial bleeding with ticagrelor. There was, however, a significant increase in TIMI-major bleeding with both doses (60 mg and 90 mg) compared with placebo (2.3% vs. 2.6% vs. 1.06%, respectively). Dyspnoea was significantly more frequent in ticagrelor-treated patients, and this led to higher discontinuation rates. Ticagrelor 60 mg was associated with numerically lower rates of dyspnoea compared with 90 mg, but, nevertheless, resulted in discontinuation of therapy in 4.55% of cases. However, it should be borne in mind that very few patients in PEGASUS-TIMI 54 had been exposed to ticagrelor previously, so it is highly likely that lower rates of discontinuation would be seen in patients continuing ticagrelor after tolerating this for one-year post-ACS. Gout was also marginally increased in ticagrelor-treated patients, attributable to the effect of ticagrelor on serum uric acid levels.5
From the trial results overall, it is estimated that 42 major cardiovascular events per year would be prevented for every 10,000 patients treated with ticagrelor 60 mg at the cost of 31 additional non-fatal TIMI major bleeds per year.5 A more favourable net benefit might be seen when ticagrelor therapy is extended without interruption after initial one year of treatment.
Pharmacodynamic assessment showed ticagrelor 60 mg to provide potent and consistent platelet inhibition that is comparable with ticagrelor 90 mg in all compliant patients, explaining why the efficacy of the 60 mg and 90 mg doses was almost identical in PEGASUS-TIMI 54.32
DM and peripheral artery disease (PAD) patients accrued greater absolute risk reduction of MACE with ticagrelor (1.5% and 4.1%, respectively), including a 22% relative reduction in cardiovascular mortality in patients with DM.33,34 Absolute excess risk of TIMI-major bleeding was also attenuated in patients with PAD (0.12%).34 Patients with CKD (glomerular filtration rate [GFR] <60 ml/min) also had a robust absolute risk reduction of MACE (2.7%) with similar bleeding risk to those without kidney dysfunction.35
The DAPT study6 assessed the safety and efficacy of 30 months’ treatment with DAPT (aspirin + clopidogrel/prasugrel) following PCI with drug eluting stents (DES). There were 9,961 patients, who had received 12 months of DAPT post PCI with DES, randomised to continued DAPT with clopidogrel (65%) or prasugrel (35%) for a further 18 months or monotherapy with aspirin. Only 26% of patients had PCI for MI and the trial excluded patients with MACE or significant bleeding in the 12-month period post-PCI. Prolonged DAPT resulted in a reduction of definite stent thrombosis (0.3% vs. 1.2%) and MACE (4.3% vs. 5.9%, p<0.001).6 This was primarily driven by a reduction in MI, both related and unrelated to stent thrombosis. There was no effect on cardiovascular mortality, but all-cause mortality was numerically higher in the continued therapy group (2% vs. 1.5%, p=0.05), mainly due to excess non-cardiovascular mortality with prolonged therapy.
Prolonged thienopyridine-based DAPT had previously been assessed in multiple smaller trials with no clear benefit of prolonged therapy.36-38 However, a collaborative meta-analysis that included the subgroups with an index event of MI and the 21,162 patients from the PEGASUS-TIMI 54 study showed that prolonged therapy reduced MACE (6.4% vs. 7.5%, p=0.001) and reduced cardiovascular death (2.3% vs. 2.6%, p=0.03) with no significant increase in non-cardiovascular death.39
Shorter duration DAPT
In cases of heightened bleeding risk, or when interruption to DAPT is anticipated, it might become necessary to stop DAPT. Trials assessing short durations of DAPT (three to six months) post-PCI with modern second-generation DES have shown comparable safety with 12 months’ therapy.40,41 Furthermore, polymer-free DES were superior to bare-metal stents with one-month DAPT post-PCI.42 These trials, however, have primarily recruited low-risk stable patients and were designed to confirm the safety of these devices in terms of late stent thrombosis. They have also used clopidogrel-based DAPT and, therefore, the results cannot be generalised to ticagrelor-based DAPT in patients with ACS.
Practical considerations and conclusions
Due to clopidogrel’s limitations and the superior efficacy of ticagrelor and prasugrel in their respective trials, European Society of Cardiology (ESC) NSTE-ACS 2015 and STE-ACS 2012 guidelines give a class I recommendation for 12 months of DAPT, giving preference to the potent P2Y12 inhibitors in the absence of contraindications or requirement for oral anticoagulant therapy.17,43 Prasugrel, however, is only indicated in STE-ACS patients undergoing primary PCI and only post-angiography in other ACS patients if PCI is indicated. Furthermore, prasugrel is not recommended for patients >75 years of age, <60 kg or with a history of cerebrovascular disease. Ticagrelor, on the other hand, is preferred to clopidogrel regardless of treatment strategy in patients with MI and moderate- to high-risk unstable angina. Ticagrelor should ideally be administered on presentation, as was done in the PLATO trial, although delaying therapy may be appropriate if patients are planned for immediate coronary angiography in centres that have short waiting times for CABG surgery. Clopidogrel continues to play a role in stable coronary artery disease patients undergoing PCI, ACS patients with contraindications to ticagrelor/prasugrel (e.g. history of intracranial haemorrhage) and those who require concurrent oral anticoagulant therapy.44
Although the ESC NSTE-ACS 2015 guidelines were prepared prior to the publications of the subgroup analyses of the PEGASUS-TIMI 54 trial, they give a class IIb indication for prolonged therapy after careful assessment of ischaemic and bleeding risk.17
The National Institute for Health and Care Excellence (NICE) have considered and issued recommendations concerning the cost-effectiveness of extended ticagrelor-based DAPT post-ACS. Estimated costs per gained quality-adjusted life year (QALY) ranged between £20,000 and £30,000. On this basis, NICE has concluded that extending ticagrelor therapy by up to three years after the first year of treatment is cost-effective.45
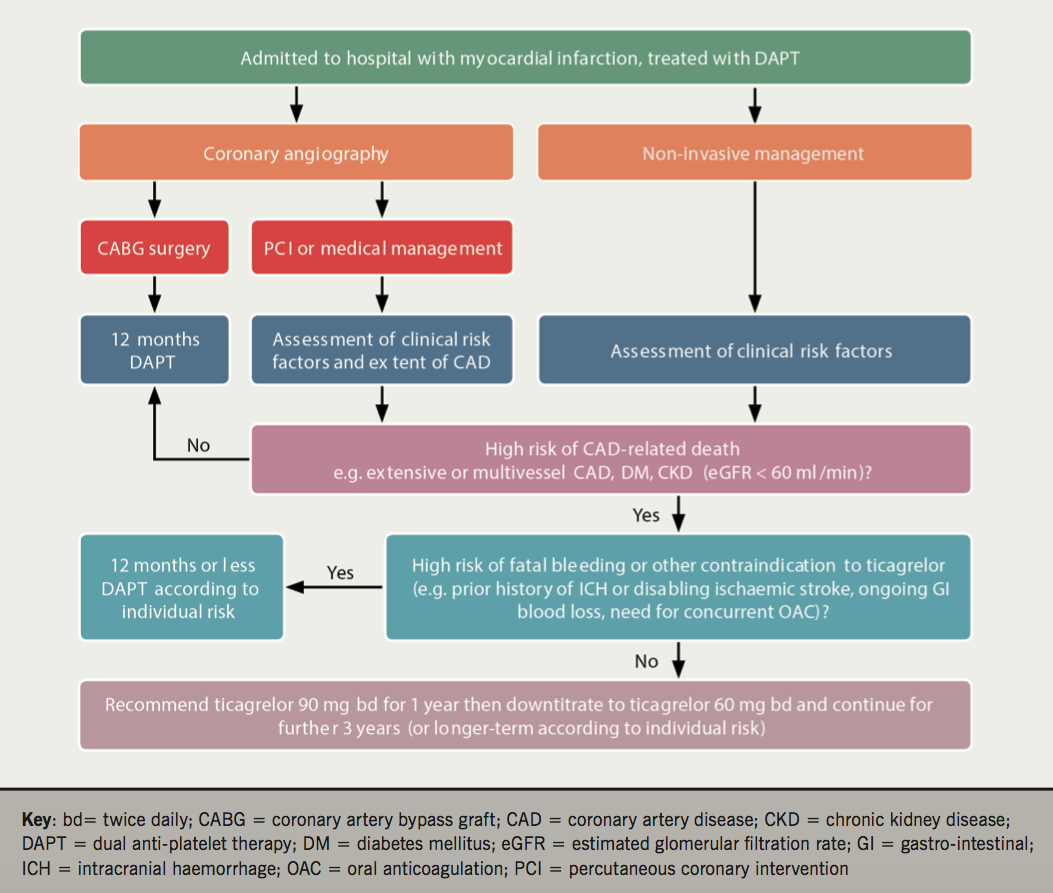
In our opinion, ticagrelor-based prolonged DAPT should be considered in patients with MI and other high-risk features (e.g. CKD not requiring dialysis, multiple MIs, extensive coronary artery disease or DM). In the PEGASUS-TIMI 54 trial, patients who had recently discontinued DAPT (<30 days) had higher rates of MACE and ticagrelor resulted in greater benefit in this group of patients.33 We, therefore, propose a strategy to down-titrate ticagrelor to 60 mg twice daily 12 months post-ACS in suitable patients, as opposed to searching for stable patients on aspirin monotherapy (figure 1). The decision to use extended ticagrelor therapy can be made prior to hospital discharge for ACS, on the basis of clinical risk factors and extent of coronary artery disease, and then reviewed at any stage thereafter, should issues with tolerability arise. This decision can be conveyed to the general practitioner in discharge correspondence with a recommendation to down-titrate the dose of ticagrelor from 90 mg to 60 mg twice daily at 12 months (figure 1).
Conflict of interest
WS: none declared. RFS has received institutional research grants from AstraZeneca and PlaqueTec; consultancy fees from Actelion, AstraZeneca, Avacta, Bayer, Bristol Myers Squibb/Pfizer, Novartis, The Medicines Company, PlaqueTec and ThermoFisher Scientific; and speaker fees from AstraZeneca.
Key messages
- Ticagrelor or prasugrel are recommended for one year after myocardial infarction (MI), in preference to clopidogrel, in patients without contraindications or requiring oral anticoagulant therapy
- Long-term risks of death due to ischaemic or bleeding events should be considered in MI patients prior to hospital discharge
- Prolonged dual antiplatelet therapy beyond one year is appropriate for patients at high risk of coronary artery disease-related death, in the absence of high risk of fatal bleeding
- For ticagrelor-treated MI patients, a recommendation can be made at hospital discharge to downtitrate from 90 mg to 60 mg bd at one year, continued for a further three years or long term according to individual risk.
References
1. Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med 1984;310:1137–40. https://doi.org/10.1056/NEJM198405033101801
2. Storey RF. Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharm Des 2006;12:1255–9. https://doi.org/10.2174/138161206776361318
3. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30–47. https://doi.org/10.1038/nrcardio.2014.156
4. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–70. https://doi.org/10.1093/eurheartj/ehu505
5. Bonaca MP, Bhatt DL, Cohen M et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. https://doi.org/10.1056/NEJMoa1500857
6. Mauri L, Kereiakes DJ, Yeh RW et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–66. https://doi.org/10.1056/NEJMoa1409312
7. Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost 1999;25(suppl 2):25–8.
8. Stone GW, Witzenbichler B, Weisz G et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 2013;382:614–23. https://doi.org/10.1016/S0140-6736(13)61170-8
9. Yusuf S, Zhao F, Mehta SR et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. https://doi.org/10.1056/NEJMoa010746
10. Mehta SR, Yusuf S, Peters RJ et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527–33. https://doi.org/10.1016/S0140-6736(01)05701-4
11. Sabatine MS, Cannon CP, Gibson CM et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179–89. https://doi.org/10.1056/NEJMoa050522
12. Chen ZM, Jiang LX, Chen YP et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21. https://doi.org/10.1016/S0140-6736(05)67660-X
13. Payne CD, Li YG, Small DS et al. Increased active metabolite formation explains the greater platelet inhibition with prasugrel compared to high-dose clopidogrel. J Cardiovasc Pharmacol 2007;50:555–62. https://doi.org/10.1097/FJC.0b013e3181492209
14. Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. https://doi.org/10.1056/NEJMoa0706482
15. Roe MT, Armstrong PW, Fox KA et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. https://doi.org/10.1056/NEJMoa1205512
16. Montalescot G, Bolognese L, Dudek D et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010. https://doi.org/10.1056/NEJMoa1308075
17. Roffi M, Patrono C, Collet JP et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. https://doi.org/10.1093/eurheartj/ehv320
18. Gurbel PA, Bliden KP, Butler K et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009;120:2577–85. https://doi.org/10.1161/CIRCULATIONAHA.109.912550
19. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos 2010;38:1514–21. https://doi.org/10.1124/dmd.110.032250
20. Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. https://doi.org/10.1056/NEJMoa0904327
21. Storey RF, Becker RC, Harrington RA et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J 2011;32:2945–53. https://doi.org/10.1093/eurheartj/ehr231
22. Storey RF, Bliden KP, Patil SB et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol 2010;56:185–93. https://doi.org/10.1016/j.jacc.2010.01.062
23. Storey RF, Becker RC, Harrington RA et al. Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] pulmonary function substudy). Am J Cardiol 2011;108:1542–6. https://doi.org/10.1016/j.amjcard.2011.07.015
24. Steg PG, James S, Harrington RA et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 2010;122:2131–41. https://doi.org/10.1161/CIRCULATIONAHA.109.927582
25. James S, Angiolillo DJ, Cornel JH et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2010;31:3006–16. https://doi.org/10.1093/eurheartj/ehq325
26. James S, Budaj A, Aylward P et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010;122:1056–67. https://doi.org/10.1161/CIRCULATIONAHA.109.933796
27. Husted S, James S, Becker RC et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes 2012;5:680–8. https://doi.org/10.1161/CIRCOUTCOMES.111.964395
28. Mahaffey KW, Wojdyla DM, Carroll K et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2011;124:544–54. https://doi.org/10.1161/CIRCULATIONAHA.111.047498
29. van Giezen JJ, Sidaway J, Glaves P, Kirk I, Bjorkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther 2012;17:164–72. https://doi.org/10.1177/1074248411410883
30. Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther 2014;19:209–19. https://doi.org/10.1177/1074248413511693
31. Sumaya W, Storey RF. Ticagrelor: effects beyond the P2Y12 receptor. Interv Cardiol Clin 2017;6:49–55. https://doi.org/10.1016/j.iccl.2016.08.004
32. Storey RF, Angiolillo DJ, Bonaca MP et al. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS-TIMI 54 trial. J Am Coll Cardiol 2016;67:1145–54. https://doi.org/10.1016/j.jacc.2015.12.062
33. Bhatt DL, Bonaca MP, Bansilal S et al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732–40. https://doi.org/10.1016/j.jacc.2016.03.529
34. Bonaca MP, Bhatt DL, Storey RF et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719–28. https://doi.org/10.1016/j.jacc.2016.03.524
35. Magnani G, Storey RF, Steg G et al. Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. Eur Heart J 2016;37:400–08. https://doi.org/10.1093/eurheartj/ehv482
36. Bhatt DL, Fox KA, Hacke W et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. https://doi.org/10.1056/NEJMoa060989
37. Collet JP, Silvain J, Barthelemy O et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577–85. https://doi.org/10.1016/S0140-6736(14)60612-7
38. Valgimigli M, Campo G, Monti M et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015–26. https://doi.org/10.1161/CIRCULATIONAHA.111.071589
39. Udell JA, Bonaca MP, Collet JP et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–9. https://doi.org/10.1093/eurheartj/ehv443
40. Gwon HC, Hahn JY, Park KW et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505–13. https://doi.org/10.1161/CIRCULATIONAHA.111.059022
41. Kim BK, Hong MK, Shin DH et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340–8. https://doi.org/10.1016/j.jacc.2012.06.043
42. Urban P, Meredith IT, Abizaid A et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–47. https://doi.org/10.1056/NEJMoa1503943
43. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. https://doi.org/10.1093/eurheartj/ehs215
44. Lip GY, Windecker S, Huber K et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014;35:3155–79. https://doi.org/10.1093/eurheartj/ehu298
45. National Institute for Health and Care Excellence. Ticagrelor for preventing atherothrombotic events after myocardial infarction. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/ta420/chapter/3-Evidence [accessed 9 May 2017].
close window and return to take test
All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.



