A recent survey of healthcare professionals confirms that hyperkalaemia is considered as a common and important clinical issue for patients receiving renin-angiotensin-aldosterone-system (RAAS) inhibitors in particular. Successful interventions to manage hyperkalaemia appear beneficial rather than avoidance or dose reduction of these RAAS inhibitors in patients with chronic heart failure, diabetic nephropathy or prior myocardial infarction.
Two newer potassium exchange resins, patiromer and sodium zirconium cyclosilicate (ZS-9), may offer improved predictability, tolerability, and efficacy for managing patients with hyperkalaemia.
Introduction
Modulation of the RAAS is an integral part of the management for patients with chronic heart failure, prior myocardial infarction and diabetic nephropathy. Evidence from large scale trials demonstrates the clear prognostic benefit of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists and more recently angiotensin receptor neprilysin inhibitors for these high-risk groups.1-3 The use of these agents, particularly in combination, can be associated with hyperkalaemia, although the incidence is unknown.4 A clear trend exists between the development of hyperkalaemia and declining renal function.5 The Renal Association recommends adopting the definition of hyperkalaemia by the European Resuscitation Council Guideline, with hyperkalaemia being stratified as mild (5.5–5.9 mmol/L), moderate (6.0–6.4 mmol/L) or severe (≥6.5 mmol/L).6 The European Society of Cardiology (ESC) guideline for the diagnosis and treatment of acute and chronic heart failure defines acute hyperkalaemia as >6.0 mmol/L.7
Whilst intuitively hyperkalaemia may present a clinical management issue for patients with chronic kidney disease (CKD) or chronic heart failure, the precise impact on optimisation of RAAS blockers is uncertain. The Assessment of Treatment with Lisinopril and Survival (ATLAS) study group suggest that low doses of the ACE inhibitor lisinopril may provide approximately half of the benefits associated with the use of high doses of this drug.8 Clear recommendations are made in the ESC guideline that ACE inhibitors should be up-titrated to maximum tolerated evidence-based doses.7 In clinical practice maximal therapy may be limited because of clinical variables such as worsening renal function, symptomatic hypotension and hyperkalaemia. For example, problematic hyperkalaemia was identified as an issue following publication of the Randomized Aldactone Evaluation Study (RALES), which showed prognostic benefit of spironolactone in severe heart failure.1 A subsequent publication in 2004 examined the relationship between rate of spironolactone prescriptions and hospitalisation for hyperkalaemia in ambulatory patients before and after RALES. It demonstrated an increase in spironolactone prescriptions and hyperkalaemia- associated morbidity and mortality following the publication of RALES in 1999. The occurrence of this complication can however be reduced with close monitoring and appropriate treatment of hyperkalaemia.9
In view of the long-term prognostic benefits for RAAS-blocking therapies, successful interventions to manage hyperkalaemia might be perceived as beneficial, as they may prevent the need of avoidance, or dose reduction, of RAAS-blockade in certain patients. This is particularly so for patients with high-risk conditions such as chronic heart failure (reduced ejection fraction), diabetic CKD, and patients with prior myocardial infarction where these drugs have clear prognostic benefit. Yet there is a paucity of data that document how hyperkalaemia is managed in routine clinical practice, and its impact on subsequent RAAS blocker use.
Healthcare professional survey
In order to understand whether healthcare professionals perceive hyperkalaemia as a significant clinical issue and if so whether it impacts on RAAS blockade, the Cardiorenal Forum (www.cardiorenalforum.com) conducted a UK-focused survey. Healthcare practitioners from The British Journal of Cardiology and the Cardiorenal Forum databases participated in an anonymised online survey.10 Participants were asked to complete 14 questions designed to assess the impact that hyperkalaemia has on their individual prescribing of RAAS blockade. The questions also enquired whether individuals were influenced by guidelines when hyperkalaemia is identified, and if these altered their clinical management.
Complete and independent responses were received from 112 professionals (37% had attended the Cardiorenal Forum) involved in the care of patients with cardiorenal conditions. Although the majority of respondents were from the UK (81%), there was representation from Europe and other countries. Most respondents were medically trained doctors (73/112 respondents); 30/112 were nurses, 4/112 were clinical pharmacists and 5/112 were ‘other’ practitioners. The number of consultant doctors was 42 (37.5% of all respondents) with 31 (27.7%) non-consultant doctors (26 training grades and 5 GPs). Around three quarters of individuals worked in a hospital environment with a greater proportion specialising in cardiac care (55.4%). Renal specialists (24.1%) were also represented, along with other areas of clinical medicine.
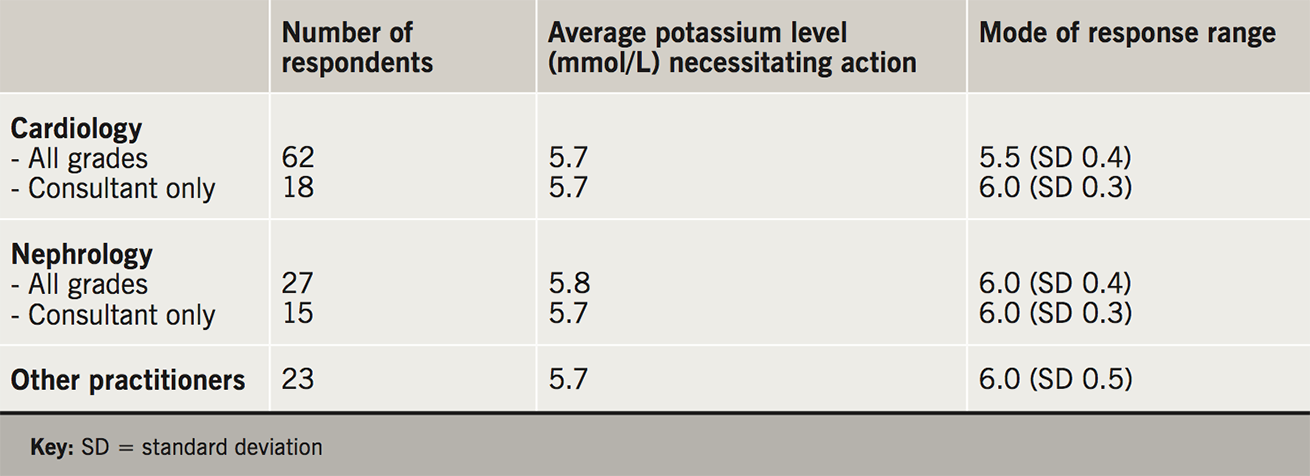
Across all respondents, the serum potassium level cited as requiring treatment ranged from 4.8 to 6.6 mmol/L (mean 5.7 mmol/L). There was little difference in viewpoint between respondents in cardiology and nephrology (table 1). However, higher values of hyperkalaemia prompting treatment were more likely to be cited by nephrologists. The reasons given for consideration of intervention at an earlier level of potassium included concerns regarding cardiac stability (31.5%) and deterioration in renal function (15.7%).
In this survey, the majority of professionals (70%) felt that hyperkalaemia would affect the use of RAAS blocking medications in up to a quarter of their patients, with the minority (10%) having this concern for over half of their patient population. The indication for mineralocorticoid antagonist (MRA) use was commonly heart failure, but also blood pressure control and proteinuria.
Published guidelines are likely to influence RAAS inhibitor and MRA use. The ESC heart failure guideline7 and the National Institute for Health and Care Excellence (NICE) guideline on chronic kidney disease in adults: assessment and management (Clinical guideline [CG182])11 are commonly cited tools in the support of treatment with RAAS blockers. From the survey, the ranking of different guidelines influencing the choice of RAAS inhibitor and MRA management according to priority given was as follows (likely to be influenced by specialty of the respondent):
- ESC heart failure guideline7
- NICE chronic kidney disease in adults: assessment and management clinical guideline [CG182]11
- Locally published protocols
- The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI)12
- Other guidelines e.g. Scottish Intercollegiate Guidelines Network (SIGN) chronic heart failure guideline13 and NICE chronic heart failure guideline in adults.14
When considering which factors influence the initiation and choice of RAAS inhibitor, the priority was:
- renal function
- cardiac function
- blood pressure
- previous adverse effect of RAAS inhibition
- previous episodes of hyperkalaemia
- diabetes or impaired glucose tolerance
- previous episodes of hypotension.
As such, it appears that whilst long-term benefits are proven for RAAS inhibitor therapies, there is a recognition that hyperkalaemia may limit the use of RAAS blockers, which might impact on improving prognosis in the cardiorenal population. Across the survey, most respondents identified the need for newer agents, such as orally active potassium binders, in the management of hyperkalaemia.
Management of hyperkalaemia and novel treatments
Management of hyperkalaemia may be improved by greater awareness and the appropriate use of drugs and strategies to avert the levels that may be dangerous or life threatening. Management of hyperkalaemia will depend upon the absolute potassium value but also how acutely the elevation occurs, and includes adjustments in diet and medication, administered medication to facilitate potassium shift into cells and stabilise the cardiac membrane, and removal of potassium facilitated by diuresis, haemodialysis or oral potassium binder. Newer therapies may allow for better RAAS inhibitor tolerability and may enable higher doses to be prescribed.
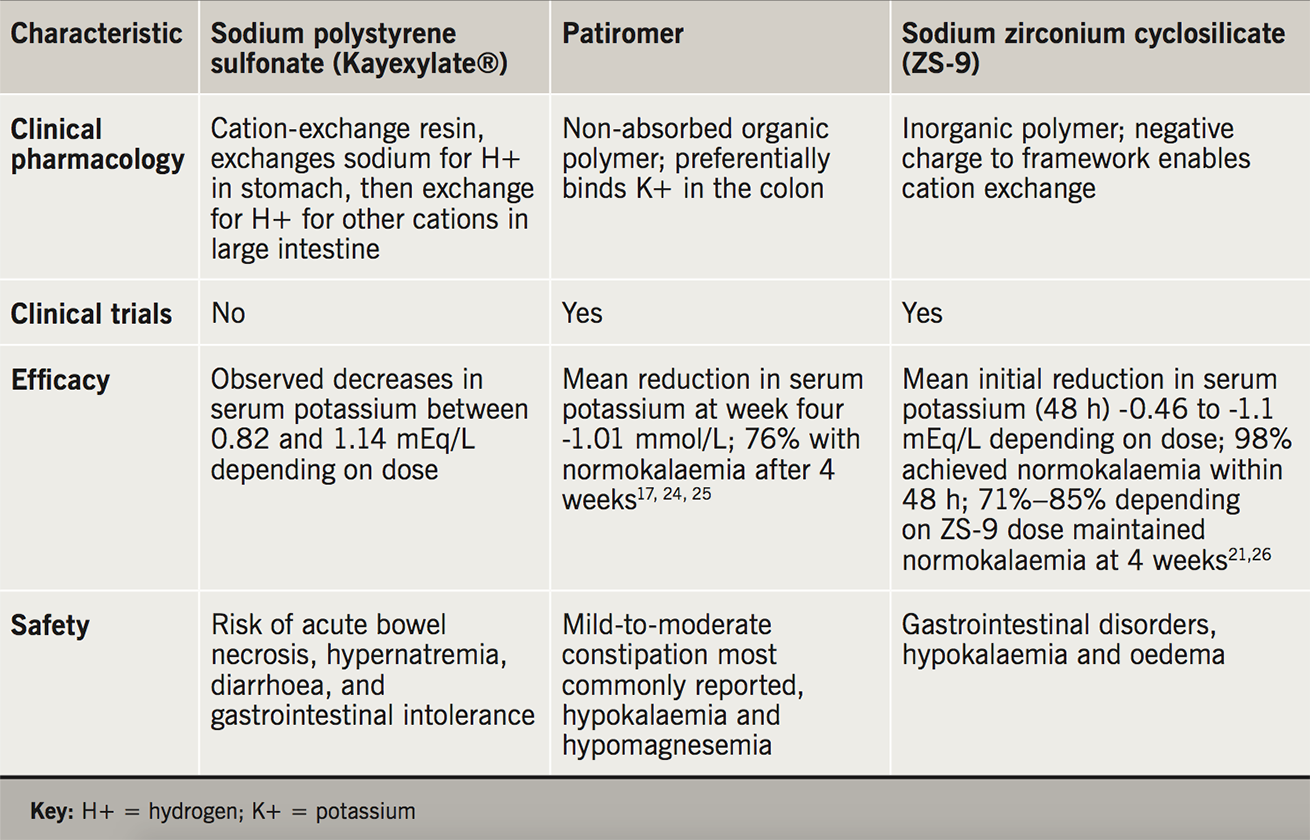
There are now three potassium-binding agents available for the management of hyperkalaemia: sodium polystyrene sulfonate (Kayexalate®, Kalexate®, Kionex®) and patiromer (Veltassa®). The third agent, sodium zirconium cyclosilicate, or ZS-9, (Lokelma®) has recently been approved in the United States and the European Union for the treatment of hyperkalaemia in adults.15
Sodium polystyrene sulfonate is a non-specific sodium-cation exchange resin. Common adverse invents including gastrointestinal disturbance (e.g. constipation, diarrhoea, nausea, vomiting, gastric irritation), electrolyte disturbance (e.g. hypokalaemia, hypomagnesemia, hypocalcaemia) and systemic alkalosis. Colonic necrosis is reported as an uncommon highly serious adverse event.15
Patiromer is an organic, non-absorbed polymer that increases faecal potassium excretion by exchanging potassium for calcium in the distal colon. It is a free-flowing, insoluble powder of small (≈100 µm) spherical beads with low viscosity.16-19 Common adverse events include gastrointestinal disturbances (like constipation, diarrhoea, nausea, vomiting, flatulence) and electrolyte abnormalities (hypokalaemia, hypercalcaemia, hypomagnesemia – these occur because the ion exchange process is non-specific). To date there have been no reported serious adverse events in the US associated with the use of patiromer.15
Sodium zirconium cyclosilicate (ZS-9) is a free-flowing, insoluble inorganic polymer, which selectively attracts potassium ions to its negatively charged crystalline lattice structure and exchanges them for sodium and hydrogen.18-21 A recent systematic review and meta-analysis of phase II and III clinical trial data to compare and contrast the efficacy and safety of patiromer and sodium zirconium cyclosilicate (ZS-9) in the treatment of hyperkalaemia has been conducted (table 2).22-3 Although the onset of action of both drugs leads to significant reduction in hyperkalaemia within the first few hours, the meta-analysis concluded that sustained normokalaemia was more favourable with patiromer use in the longer term. Additionally, ZS-9 was associated with an increased incidence of urinary tract infections and oedema (because of the sodium exchange), whereas patiromer was associated with more gastrointestinal upset.22-3 Although head-to-head trials of patiromer or ZS-9 versus sodium polystyrene sulfonate have not been conducted, the theoretical advantages of the new agents over existing therapy are apparent (table 2).
Clinical trials
Patiromer
The PEARL-HF study evaluated the use of patiromer in patients with heart failure with concomitant use of spironolactone. PEARL-HF was a double-blind, placebo controlled trial in which 105 patients with a baseline potassium of 4.3–5.4 mmol/L and heart failure (New York Heart Association class II-III) were treated with spironolactone in addition to standard therapy and were randomised to patiromer (30 g) or placebo for four weeks. The primary end point was mean change of serum potassium from baseline to 28 days. Results indicated that patiromer significantly reduced serum potassium levels in comparison to placebo at 28 days and facilitated a higher proportion of patients with heart failure to receive 50 mg/day of spironolactone (91% patiromer vs. 74% placebo, P=0.019).17 A small but statistically significant decrease in magnesium levels were observed in the patiromer group (-0.22 mg/dL in patiromer group versus 0.01 mg/dL in placebo group). This was not felt to be of clinical significance. In general, the drug was well tolerated except for transient gastro-intestinal symptoms like flatulence, diarrhoea, constipation and vomiting.17
Patiromer has also been trialed in patients with diabetes and renal impairment (AMETHYST-DN).24 All 306 enrolled patients were on RAAS inhibitors with a history of type 2 diabetes and hyperkalaemia. In the trial (treatment for 52 weeks), patients were stratified to receive one of three starting doses of patiromer depending on whether they had mild or moderate hyperkalaemia. Patiromer was titrated to achieve a serum potassium level of 5.0 mmol/L or below. There was no placebo arm. Overall statistically significant reductions in potassium were seen in all treatment arms (0.35 to 0.97 mEq/l, varying according to the dose of patiromer that had been administered). An increase in potassium was seen on cessation of the drug.
The OPAL-HK was of similar trial design; all 243 patients were receiving RAAS inhibitors at baseline and they had common comorbidities associated with renal impairment and hypertension. This was a two-phase randomised control trial which evaluated the efficacy of patiromer against placebo. In the first phase, patients with potassium 5.1–6.5 mmol/L were treated with patiromer for four weeks. Those patients whose potassium fell to 5.1 mmol/L or less entered the second phase in which they were randomised to continue patiromer or switch to placebo. Of the 107 patients so randomised, the recurrence of hyperkalaemia was 60% in the placebo arm but only 15% in those receiving patiromer.25 Patiromer, although well tolerated, was associated with constipation. Changes in magnesium were similar to those observed in other patiromer studies.
Sodium zirconium cyclosilicate (ZS-9)
The efficacy of ZS-9 has been evaluated in 753 patients with significantly reduced renal function (dialysis patients excluded) and hyperkalaemia.21 This study captured a wide range of patients with varying comorbidities and including some without RAAS inhibitor use. The trial was designed as a two-part, randomised, double-blind, placebo-controlled study. The intervention with ZS-9 involved randomisation to one of three different dose regimes or to a placebo group. The sub-acute phase involved randomised withdrawal. The achievement of normokalaemia (3.5–4.9 mmol/L) within 48 hours of therapy was significant as compared to placebo. Gastrointestinal side-effects, such as constipation were most common. However, in contrast to studies with patiromer there were no reports of hypomagnesemia, although this finding was not significant.21
The Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance (HARMONIZE) study evaluated ZS-9 in 258 patients including those with renal impairment (excluding dialysis dependent patients) in a two-phase design.26 Using 10 g thrice daily of ZS-9, potassium fell from 5.6 to 4.5 mmol/L at a median interval of just 2.2 hours. In the second phase, patients were randomised to three different doses of ZS-9 or placebo. At 28 days the proportions of patients maintaining potassium <5.1 mmol/L was 80–94% in the ZS-9 treated patients compared to just 46% in the placebo group. No clinically significant changes in serum magnesium, phosphate, or bicarbonate were observed. Adverse events were similar in the two arms, but more oedema was seen with the highest of the three doses of ZS-9, and there was a higher rate of hypokalaemia with the two higher doses of ZS-9.
Conclusion and future perspectives
Hyperkalaemia is considered by healthcare professionals as a common and important clinical issue for patients receiving RAAS blockers. In view of the prognostic benefit for RAAS-blocking therapies, successful interventions to manage hyperkalaemia would appear beneficial rather than the alternative approach which would be avoidance or dose reduction of the drugs in patients with chronic heart failure, diabetic nephropathy or prior myocardial infarction.27-9 Non-adherence results in a substantial risk of recurrent hyperkalaemia.21,24-6
The newer potassium exchange resins may well offer improved predictability, tolerability, and efficacy for managing hyperkalaemia in patients. The true burden of hyperkalaemia in clinical practice and whether better control of potassium will permit continuation of (or higher doses) of RAAS blockers remains uncertain. For example, even after optimisation of potassium control, patients might still have other issues such as hypotension or worsening renal function. Ongoing studies will help define future guidelines.
Key messages
- Hyperkalaemia is considered by healthcare professionals as a common and important clinical issue for patients receiving renin angiotensin aldosterone inhibitors
- Successful interventions to manage hyperkalaemia appear beneficial rather than avoidance or dose reduction of these drugs in patients with chronic heart failure, diabetic nephropathy or prior myocardial infarction
- Newer potassium exchange resins may offer improved predictability, tolerability, and efficacy for managing hyperkalaemia
Conflict of interest
Vifor Pharma UK provided an unrestricted grant to The British Journal of Cardiology and the Cardiorenal Forum for conducting and analysis of the healthcare professionals survey.
NK and SB: none declared. PAK has received honoraria for talks and advisory boards from Vifor Fresenius. PRK has received speaker fees and honoraria from Vifor Pharma and AstraZeneca.
References
1. Pitt B, Zannad F, Remme WJ et al. for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17. https://doi.org/10.1056/NEJM199909023411001
2. Group TCTS. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429–35.
3. McMurray JJV, Packer M, Desai AS et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. https://doi.org/10.1056/NEJMoa1409077
4. Tamirisa KP, Aaronson KD, Koelling TM. Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure. Am Heart J 2004;148:971–8. https://doi.org/10.1016/j.ahj.2004.10.005
5. Sica DA, Gehr TW, Yancy C. Hyperkalemia, congestive heart failure, and aldosterone receptor antagonism. Congest Heart Fail 2003;9:224–9.
6. UK Renal Association. Clinical Practice Guidelines, Treatment Of acute hyperkalaemia In adults. UK Renal Association 2014. https://renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.pdf
7. Ponikowski P, Voors AA, Anker SD et al. 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Failure 2016;18. https://doi.org/10.1016/j.rec.2016.11.005
8. Packer M, Poole-Wilson P, Armstrong PW et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100(23):2312–8.
9. Juurlink DN, Mamdani MM, Lee DS et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004;351:543–51. https://doi.org/10.1056/NEJMoa040135
10. Kalsi N, Purcell H, Kalra P, Kalra PR. Impact of hyperkalaemia on managing patients with cardiorenal disease; a healthcare professional prospective. Eur J Heart Fail 2018;20 (suppl S1):i817 (abstract). https://doi.org/10.1002/ejhf.1197
11. National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. Clinical guideline [CG182]. London: NICE, July 2014 (Last updated January 2015). https://www.nice.org.uk/guidance/cg182
12. National Kidney Foundation. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI)™. www.kidney.org
13. Scottish Intercollegiate Guidelines Network (SIGN). SIGN 147. Management of chronic heart failure. A national clinical guideline. March 2016. Edinburgh: SIGN; 2016 (publication no. 147). http://sign.ac.uk
14. National Institute for Health and Care Excellence (NICE). Chronic heart failure in adults: management. Clinical guideline (CG108), August 2010. London: NICE; 2010. https://www.nice.org.uk/guidance/cg108
15. Chaitman M, Dixit B, Bridgman MB. Potassium-binding agents for the clinical management of hyperkalemia. Pharm Therapeut. 2016;41(1):43–50.
16. Weir MR, Bakris GL, Bushinsky DA et al. OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211– 21. https://doi.org/10.1056/NEJMoa1410853
17. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011;32:820–8. https://doi.org/10.1093/eurheartj/ehq502
18. McCullough PA, Beaver TA, Bennett-Guerrero E et al. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med 2014;15(1):11–23.
19. Buysse JM Huang IZ, Pitt B. PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol 2012;8:17–28. https://doi.org/10.2217/fca.11.71
20. Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One 2014;9(12):e114686. https://doi.org/10.1371/journal.pone.0114686
21. Packham DK, Rasmussen HS, Lavin PT et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015;372:222–31. https://doi.org/10.1056/NEJMoa1411487
22. Tamargo J, Caballera R, Delpón E. New drugs for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors – hype or hope? Discov Med 2014;18:249–54.
23. Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017;37:401–11. https://doi.org/10.1002/phar.1906
24. Bakris GL, Pitt B, Weir MR et al.; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN Randomized Clinical Trial. JAMA 2015;314:151–61. https://doi.org/10.1001/jama.2015.7446
25. Weir MR, Bakris G, Bushinsky DA et al.; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;15:211–21. https://doi.org/10.1056/NEJMoa1410853
26. Kosiborod M, Rasmussen HS, Lavin P et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE Randomized Clinical Trial. JAMA 2014;321:2223–33. https://doi.org/10.1001/jama.2014.15688
27. Levin A, Smith PE, Bilous RW et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 2013;3(1):1–150. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf
28. James PA, Oparil S, Carter BL et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. https://doi.org/10.1001/jama.2013.284427
29. American Diabetes Association. Cardiovascular disease and risk management. Diabet Care 2015;38(suppl 1):S49–S57. https://doi.org/10.2337/dc15-S011
