Introduction
Device therapy has revolutionised the landscape of heart failure over the past 10 years. Prior to device therapy, the most important trials in heart failure (HF) management centred on pharmacotherapy. The CONSENSUS (Cooperative North Scandinavian Enalapril Survival Study) trial (1987),1 showed the importance of optimal blockade of the renin–angiotensin–aldosterone system (RAAS). Similarly, CIBIS-II (Cardiac Insufficiency Bisoprolol Study II) (1999)2 and RALES (Randomized Aldactone Evaluation Study) (1999)3 trials did the same for beta-blockade and spironolactone, respectively.
This century, device therapy has also become part of the mainstream management of patients with heart failure and reduced left ventricular ejection fraction (HFREF). The benefits of implantable cardioverter defibrillators (ICDs) became apparent in 2005 with the SCD-HEFT (Sudden Cardiac Death in Heart Failure Trial)4 trial showing that ICD implantation in patients with New York Heart Association (NYHA) II/III and left-ventricular ejection fraction (LVEF) <35% reduced mortality compared with both amiodarone and placebo. The COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure)5 trial subsequently showed clear morbidity and mortality benefits of cardiac resynchronisation therapy, with or without a defibrillator, compared with medical therapy in patients with severe HF (NYHA III/IV) and broad QRS width. The benefits of cardiac resynchronisation therapy alone in the subset of patients with a broad QRS and left bundle branch block was demonstrated further in the CARE-HF (Cardiac Resynchronization in Heart Failure)6 study. The landmark CARE-HF study showed that a cardiac resynchronisation pacemaker (CRTP) reduces all-cause mortality, improves quality of life, reduces HF hospitalisations and results in favourable left ventricular remodelling. However, it is important to note that device therapy, although unequivocally beneficial, is in addition to optimal pharmacological therapy, and not an alternative.
Defining ‘optimal’ medical therapy
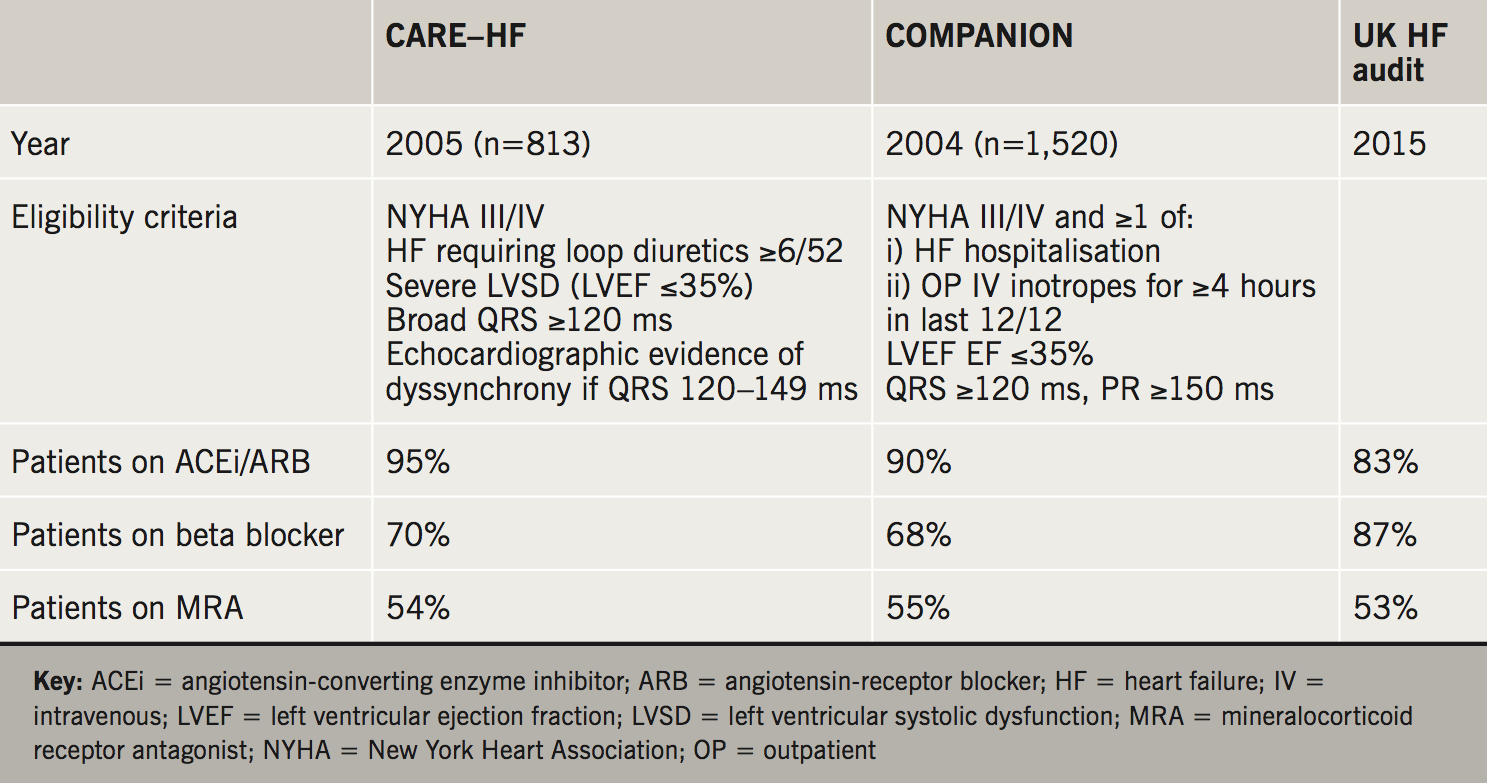
The proportion of patients receiving the three classes of pharmacotherapy proven to improve morbidity and mortality in HF in the two major CRT trials are summarised in table 1. In this setting, the proportion of patients receiving an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB) was high (90–95%); comparing favourably with the most recent UK HF audit data (83%). However, fewer patients received a beta blocker (68–70% vs. 83%) and mineralocorticoid antagonist (MRA) use was low (54–55%). The trial design described ‘standard pharmacological therapy’ as baseline, perhaps slightly different to optimal. Nevertheless, given that all three classes have demonstrated summative benefit in HF, and should be used in conjunction in appropriate patients (i.e. those in these trials), there was clearly room for increased medical therapy. This is a simple and relatively risk-free step and perhaps one that needs to be explored further in clinical practice prior to opting for device therapy.
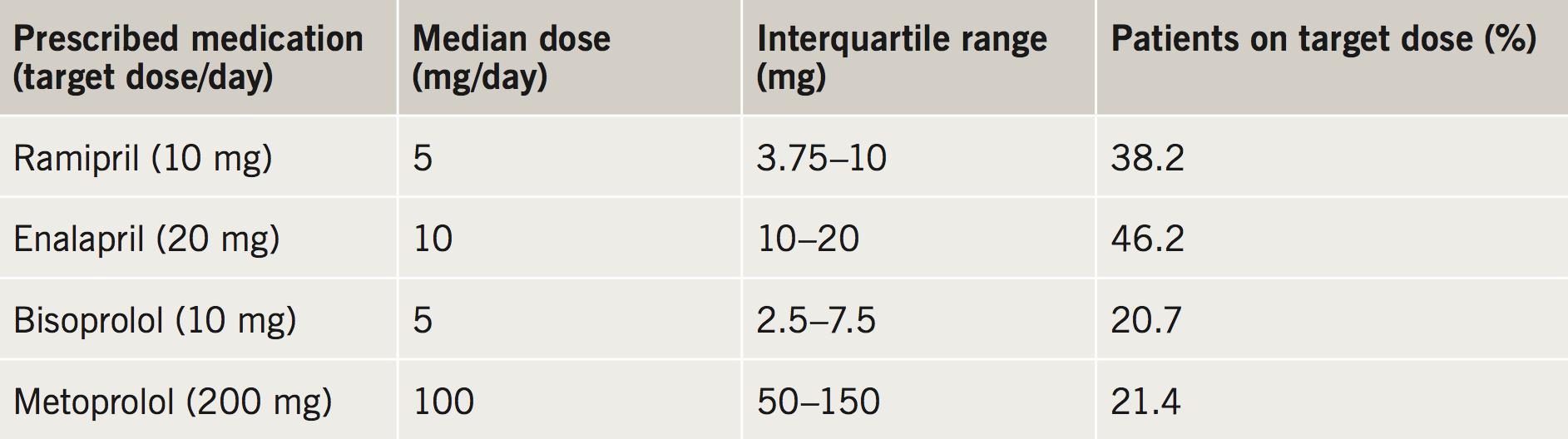
Delving further into CARE-HF and COMPANION trials it is clear that pharmacotherapy strategies are presented in a binary fashion. Patients included in the trials were either listed as being treated with a class of drugs or not. The doses achieved are not widely reported. Table 2 summarises data collected by the 2010 12-country European Survey by the European Society of Cardiology (ESC) where the median doses are compared with the target doses in HF management from the 2008 guidelines.7 Fewer than half of patients achieve the target doses, despite guidelines recommendations to up-titrate therapies to the maximal doses, in a similar fashion to the protocols used in the major trials, such as the CONSENSUS study. However, in practice, this can be difficult to achieve in all patients, due to adverse effects, such as renal insufficiency, or side effects, such as dizziness.
Angiotensin-converting enzyme inhibitors
Many studies have been performed to answer whether the beneficial effects of ACEi therapy are dose-dependent, and whether higher doses lead to better outcomes. The NETWORK8 and CHIPS (Captopril in Heart Insufficient Patients Study)9 trials examined the influence of differing doses of enalapril and captopril, respectively, on the important clinical end points, mortality and hospitalisation for heart failure. Both demonstrated trends toward improved outcomes with higher disease, although these were non-statistically significant; having been underpowered with respect to participants and event rates. The ATLAS (Assessment of Treatment with Lisinopril and Survival) study was the main trial to support higher ACEi doses, it was more robustly powered, with 3,164 patients, and a follow-up period of 46 months and showed a significant reduction in all-cause mortality and hospitalisations with a high-dose ACEi versus lower doses.10
Beta blockers
Similarly, all of the seminal beta blocker mortality trials, CIBIS-II,2 MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure)11 and COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival),12 used protocols that included a slow upward titration to a target dose, as tolerated. Despite this, only limited numbers, ranging from 22% to 53%, achieved target doses, yet the mortality benefit was still present. Later subgroup analysis of the CIBIS-II data was carried out, whereby the subjects were divided into low-, moderate- and high-dose beta blocker groups. Though all groups showed a reduction in cardiovascular mortality, all-cause hospitalisation and cardiovascular hospitalisation, this was significantly larger in the higher and moderate dose groups compared with the low-dose group. However, the issue remains that despite higher doses showing improved outcomes, it is challenging to up-titrate beta blockers towards target doses. CIBIS-ELD (Cardiac Insufficiency Bisoprolol Study in Elderly)13 sought to evaluate doses achieved in elderly patients with congestive HF (CHF) and then force-titrate to the ESC guidelines target. In 876 patients with a mean age of 73 years, carvedilol and bisoprolol could only be titrated to the maximal recommended dose in 31%, and 55% of the patients achieved at least 50% of the target dose. There was no difference between the level of up-titration between carvedilol and bisoprolol. The main limiting factor in up-titration was low heart rate. However, despite the suboptimal level of optimisation, there was a significant improvement in NYHA class, six-minute walk distance and LVEF. In the longer-term follow-up of a subset of the CIBIS–ELD group, the reduction in mortality and HF-related adverse events was associated with a lowering of heart rate to the range 55–64 bpm, and did not correlate with beta blocker dosage.14 This raises the interesting possibility that titration of pharmacotherapy should be determined by physiological parameters.
Mineralocorticoid antagonist (MRA)
The EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) study looked at the addition of eplerenone to optimal pharmacological therapy in patients with LVEF <35% but with only mild heart failure symptoms (NYHA II).15 The dose was titrated from 25 mg to 50 mg with monitoring of renal function and potassium concentrations. During the study, 60% of patients reached the maximum dose and there was a reduction in cardiovascular death and HF hospitalisation. There was also a reduction in all-cause mortality, and subsequent analysis has also shown a reduction in the incidence of new-onset atrial fibrillation.16 The proportion of patients with devices in the study was 21.7%. The mechanism by which aldosterone antagonism provides benefit is not known; it may be by reducing fibrosis and, thereby, improving favourable atrial and ventricular remodelling. There is no reason to believe that it should not provide benefit to patients with devices, but potassium concentrations should be monitored.
Therefore, while anti-failure drugs alone are beneficial in HF they need to be titrated effectively to exert maximal beneficial effect. Thus, despite the pivotal device trials receiving little emphasis to forced titration of pharmacotherapy, in clinical practice, it seems a prerequisite that robust up-titration occurs before subjecting patients to the risks and inconvenience of device implantation.
Assessment of up-titration
Trials, such as CIBIS-ELD,13 that actively tried to increase pharmacotherapy, demonstrated that these are not achieved in a large proportion (up to 50%) of patients, particularly the elderly. A pragmatic alternative to target doses, may, therefore, be ‘target effect’ assessed by biomarkers or other surrogate metrics.
Heart rate is one such potential marker to help titrate beta blockers. However, ACEi have myriad physiological effects, and it is not clear that its effects on blood pressure are directly related to its benefit in HF. As such, blood pressure may not be the most appropriate physiological biomarker for dose effect, but may still be used with tolerability in mind.
A meta-analysis of 23 HF studies in patients with HFREF, showed that improved survival correlated with a reduction in heart rate, but not the dose of beta blocker used.17 This opened up the hypothesis that heart rate reduction could be a target in patients with HFREF who were already on maximal tolerated doses of beta blockers.
The SHIFT (Systolic Heart failure treatment with the If inhibitor Ivabradine Trial)18 study used the selective sinus node inward ‘funny’ (If) channel inhibitor ivabradine in patients with HFREF on optimal medical therapy whose heart rate was >70 bpm. The results demonstrated a significant reduction in heart failure hospitalisation and heart failure mortality. Subanalyses19 illustrated a correlation between benefit and reduction in HR, further supporting the use of physiological biomarkers. An important caveat, in extrapolating the findings to patients with devices, is that the main exclusion criteria for this trial included ventricular pacing >40% or ICD shock within six months. As such, only 4% of those enrolled had CRT/ICD. Further work is, therefore, required.
Angiotensin-receptor–neprilysin inhibitors
A notable advance in recent years has been the introduction of angiotensin-receptor–neprilysin inhibitors (ARNIs). The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure)20 trial showed a clear reduction in cardiovascular mortality and HF hospitalisation with sacubitril/valsartan when compared with a higher mean dose of enalapril used in the original ACEi studies. In a large study of optimally treated HFREF patients, the trial was stopped early as it had reached its primary end point and also showed a reduction in all-cause mortality and sudden cardiac death. This resulted in a very rapid approval by the National Institute for Health and Care Excellence (NICE).21 However, this study contained only a small proportion of patients with CRT (7%) or ICD (15%), and, in the absence of subgroup analyses, it is difficult to define the additional benefit afforded to this patient group.
Does optimisation of medical therapy influence the need for device therapy?
It seems logical to assume that, if patients with HFREF were optimally medicated, the eligibility for device therapy may be modified. The use of LVEF <35% as a cut-off for device therapy means that favourable left ventricular remodelling could abrogate the need for a device, and also modify the potential mortality benefit. In patients with non-ischaemic aetiology of HFREF, primary prevention ICD did not improve all-cause mortality or cardiovascular death in a group of very well-treated patients (96% ACEi/ARB, 92% beta blocker, 58% MRA and 58% CRT).22 However, there was reduction in sudden cardiac death with the addition of an ICD. The PARADIGM-HF20 and SHIFT18 studies were published after the majority of landmark device studies. Given the considerable treatment effect of sacubitril/valsartan and ivabradine, it would be reasonable to assume that if all eligible patients were on these medications; it may reduce the number of patients that require device therapy, and may also modify the risk of mortality and even sudden cardiac death.
Modifying the clinical response following device therapy
Although device therapy leads to a clear improvement in morbidity and mortality for most patients; a significant minority derive minimal benefit and are classed as ‘non-responders’. The proportion of patients falling into this category depends on the definition of ‘response’ employed, but is generally taken to be around 30%.17 With this in mind, it is important to understand how anti-failure therapy may influence response to CRT therapy and vice versa.
A large observational study23 in 2015 showed that six months post-implantation, CRT responders (defined as improvement in NYHA class), were more likely to be on ACEi post-implantation, with a strong trend towards higher mean doses. Higher doses of ACEi and beta blocker post-CRT insertion were also associated with improved survival. Similar findings were apparent in ‘super-responders’ (defined by an EF >40%) in a related study.24 Whether improved haemodynamics after CRT insertion enable greater up-titration, or up-titration itself plays a role in aiding the response to CRT, is difficult to discern from the data. In the latter study,19 CRT non-responders were more likely to be on diuretics and digoxin pre-implantation; perhaps identifying a more advanced heart failure phenotype.
What is clear, is that continued up-titration of ACEi and beta blockers after CRT is associated with improved outcomes. It is also necessary to reassess left ventricular function following device therapy to identify individuals that still have an LVEF <35% and, therefore, would be candidates for sacubitril/valsartan.
Atrial fibrillation
Patients with atrial fibrillation (AF) were excluded from the major randomised controlled trials (CARE-HF and COMPANION). Despite this, ‘real-world’ data suggests AF occurs in a quarter of patients receiving a CRT.25 Patients with AF are more likely to be CRT non-responders and carry a worse longer-term prognosis.26 Exploration of the myriad reasons behind this is beyond the scope of this review, however, one particularly pertinent to pharmacotherapy is uncontrolled ventricular rate; which can compromise effective CRT delivery. Boriani et al.27 illustrated that the percentage of effective biventricular pacing (BiVP%) inversely correlates with ventricular rate; and that uncontrolled ventricular rate is independently associated with HF hospitalisation and death in adjusted analyses. Furthermore, in a large cohort (>30,000 patients) achieving a high BiVP% (>98.5%) was associated with reduced mortality.28 Those with AF had a worse outcome, but again, this was significantly lessened in those that could achieve a high BiVP%. Thus, in patients with devices and AF, stricter attention must be paid to adequate rate control to achieve better long-term outcomes, and referral for AV nodal ablation must be considered in those for whom pharmacological rate control has failed.
Apart from stricter rate control, a comprehensive discussion of other strategies to achieve high BiVP% is outside the scope of the present review. Current ESC guidelines propose amiodarone as first-line therapy for rhythm control strategies for patients with AF and HF.29 However, with trials such as CASTLE-AF (Catheter Ablation for Atrial Fibrillation with Heart Failure)30 showing superiority of ablation strategies over pharmacotherapy, this may change.
Integrated care for heart failure patients with devices
The volume of evidence suggests that optimising medical therapy in patients with HFREF prior to considering device therapy and after implantation is necessary and beneficial. However, it is often difficult to achieve this in clinical practice as patients may be cared for by a number of healthcare professionals, who may not have a specialist interest in HF. An integrated specialist HF pacing service has been shown to increase the number of patients on optimal doses of beta blockers and ACEi/ARB following CRT.31 However, it may not always be logistically possible to have a dedicated service, but efforts to integrate the pacing follow-up with the HF team seems to be a sensible approach to providing holistic care.
Conclusion
In the burgeoning world of device therapy, it may appear that the predominance of pharmacotherapy is dwindling. But, it is increasingly clear that to maximise benefit, the two must be used in tandem. The use of devices should be viewed as a further opportunity to review and up-titrate pharmacotherapy in our patients to improve outcomes. There are insufficient data on some newer therapies in patients with devices, and this is an area requiring further studies. Finally, more work is clearly needed to tease out how pharmacotherapy can best help the large cohort of patients that show poor response to device therapy.
Key messages
- Despite a wealth of evidence, guideline recommended doses of neurohormal blockade in heart failure with reduced ejection fraction (HFREF) is suboptimal throughout Europe
- There is room to optimise medical therapy prior to considering device therapy
- Following device therapy, further refinement of pharmacotherapy is beneficial
- An integrated approach to heart failure and device therapy may help achieve optimal care for this group of patients
Conflict of interest
BSK and CA: none declared. BC has received speaker fees from and educational support from Novartis, Servier and a number of device companies.
Articles in this supplement
Introduction
Current and future perspectives on cardiac pacing
A brief history of cardiac pacing in the UK
Cardiac resynchronisation therapy – developments in heart failure management
His-bundle pacing: UK experience and HOPE for the future!
Leadless pacing
Techniques in pacemaker and defibrillator lead extraction
Remote monitoring
Remote follow-up of ICS: a physiologist’s experience
References
1. The Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–35. https://doi.org/10.1056/NEJM198706043162301
2. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. https://doi.org/10.1016/S0140-6736(98)11181-9
3. Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–17. https://doi.org/10.1056/NEJM199909023411001
4. Bardy GH, Lee KL, Mark DB et al.; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. https://doi.org/10.1056/NEJMoa043399
5. Bristow MR, Saxon LA, Boehmer J et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. https://doi.org/10.1056/NEJMoa032423
6. Cleland JGF, Daubert J-C, Erdmann E et al.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. https://doi.org/10.1056/NEJMoa050496
7. Tavazzi L, Maggioni AP, Borer JS. Should we revise our approach to “optimal medical therapy?” The case of chronic heart failure. Eur Heart J 2013;34:2792–4. https://doi.org/10.1093/eurheartj/eht279
8. The NETWORK Investigators. Clinical outcome with enalapril in symptomatic chronic heart failure; a dose comparison. Eur Heart J 1998;19:481–9. https://doi.org/10.1053/euhj.1997.0839
9. Clement DL, De Buyzere M, Tomas M, Vanavermaete G. Long-term effects of clinical outcome with low and high dose in the Captopril in Heart Insufficient Patients Study (CHIPS). Acta Cardiol 2000;55:1–7. https://doi.org/10.2143/AC.55.1.2005711
10. Packer M, Poole-Wilson PA, Armstrong PW et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100:2312–18. https://doi.org/10.1161/01.CIR.100.23.2312
11. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–07. https://doi.org/10.1016/S0140-6736(99)04440-2
12. Packer M, Fowler MB, Roecker EB et al. Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194–9. https://doi.org/10.1161/01.CIR.0000035653.72855.BF
13. Düngen H-D, Apostolovic S, Inkrot S et al. CIBIS-ELD investigators and Project Multicentre Trials in the Competence Network Heart Failure. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail 2011;13:670–80. https://doi.org/10.1093/eurjhf/hfr020
14. Düngen H-D, Musial-Bright L, Inkrot S et al. Heart rate following short-term beta-blocker titration predicts all-cause mortality in elderly chronic heart failure patients: insights from the CIBIS-ELD trial. Eur J Heart Fail 2014;16:907–14. https://doi.org/10.1002/ejhf.121
15. Zannad F, McMurray JJV, Krum H et al. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. https://doi.org/10.1056/NEJMoa1009492
16. Swedberg K, Zannad F, McMurray JJV et al. EMPHASIS-HF Study Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol 2012;59:1598–603. https://doi.org/10.1016/j.jacc.2011.11.063
17. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009;150:784–94. https://doi.org/10.7326/0003-4819-150-11-200906020-00006
18. Swedberg K, Komajda M, Böhm M et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. https://doi.org/10.1016/S0140-6736(10)61198-1
19. Böhm M, Borer J, Ford I et al. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol 2013;102:11–22. https://doi.org/10.1007/s00392-012-0467-8
20. McMurray JJV, Packer M, Desai AS et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. https://doi.org/10.1056/NEJMoa1409077
21. National Institute for Health and Care Excellence. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. London: NICE, 2016. Available from: https://www.nice.org.uk/Guidance/TA388
22. Køber L, Thune JJ, Nielsen JC et al. DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. https://doi.org/10.1056/NEJMoa1608029
23. Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Optimization of heart failure medication after cardiac resynchronization therapy and the impact on long-term survival. Eur Heart J Cardiovasc Pharmacother 2015;1:182–8. https://doi.org/10.1093/ehjcvp/pvv016
24. Schmidt S, Hürlimann D, Starck CT et al. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J 2014;35:1051–60. https://doi.org/10.1093/eurheartj/eht514
25. Dickstein K, Bogale N, Priori S et al. Scientific Committee, National Coordinators. The European cardiac resynchronization therapy survey. Eur Heart J 2009;30:2450–60. https://doi.org/10.1093/eurheartj/ehp359
26. Tolosana JM, Hernandez-Madrid A, Brugada J et al. SPARE Investigators. Comparison of benefits and mortality in cardiac resynchronization therapy in patients with atrial fibrillation versus patients in sinus rhythm (results of the Spanish Atrial Fibrillation and Resynchronization [SPARE] Study). Am J Cardiol 2008;102:444–9. https://doi.org/10.1016/j.amjcard.2008.04.008
27. Boriani G, Gasparini M, Landolina M et al. ClinicalService cardiac centres. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail 2011;13:868–76. https://doi.org/10.1093/eurjhf/hfr046
28. Kamath GS, Cotiga D, Koneru JN et al. The utility of 12-lead Holter monitoring in patients with permanent atrial fibrillation for the identification of nonresponders after cardiac resynchronization therapy. J Am Coll Cardiol 2009;53:1050–5. https://doi.org/10.1016/j.jacc.2008.12.022
29. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. https://doi.org/10.1002/ejhf.592
30. Marrouche NF, Brachmann J, Andresen D et al. CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855
31. Guha K, Konstantinou D, Mantziari L et al. The impact of age on clinical outcomes following cardiac resynchronisation therapy. J Interv Card Electrophysiol 2014;39:95–102. https://doi.org/10.1007/s10840-013-9844-0