Introduction
Three important lines of evidence have informed the debate on optimal anticoagulation for people at risk of stroke:
- Meta-analyses have generally supported the findings from the ENGAGE-AF TIMI-481 and Hokusai-VTE2 trials, in terms of comparable efficacy and reduced bleeding risk with non-vitamin K antagonist oral anticoagulants (NOACs) versus warfarin in patients at risk of stroke, or with acute venous thromboembolism (VTE), respectively.3-8
- The randomised Birmingham Atrial Fibrillation Treatment of the Aged Study (BAFTA) trial confirmed the superiority of anticoagulation versus aspirin in elderly patients with atrial fibrillation (AF).9
- The randomised Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation trial showed that treatment with a NOAC was superior to aspirin for prevention of stroke or systemic embolism (SSE) without additional bleeding risk.10
The key lessons from these studies relate to the importance of effective anticoagulation in patients at risk of stroke. Moreover, aspirin is not a suitable substitute for treatment with a NOAC for a patient who is unsuitable to receive warfarin, and individualised treatment with a NOAC may have advantages over warfarin for appropriate patients.
This article provides a perspective from the primary-care arena on a series of important issues relating to the use of NOACs in general, and edoxaban in particular, within this setting, based on the clinical literature and the experience of the author.
Appropriate, individualised prescribing
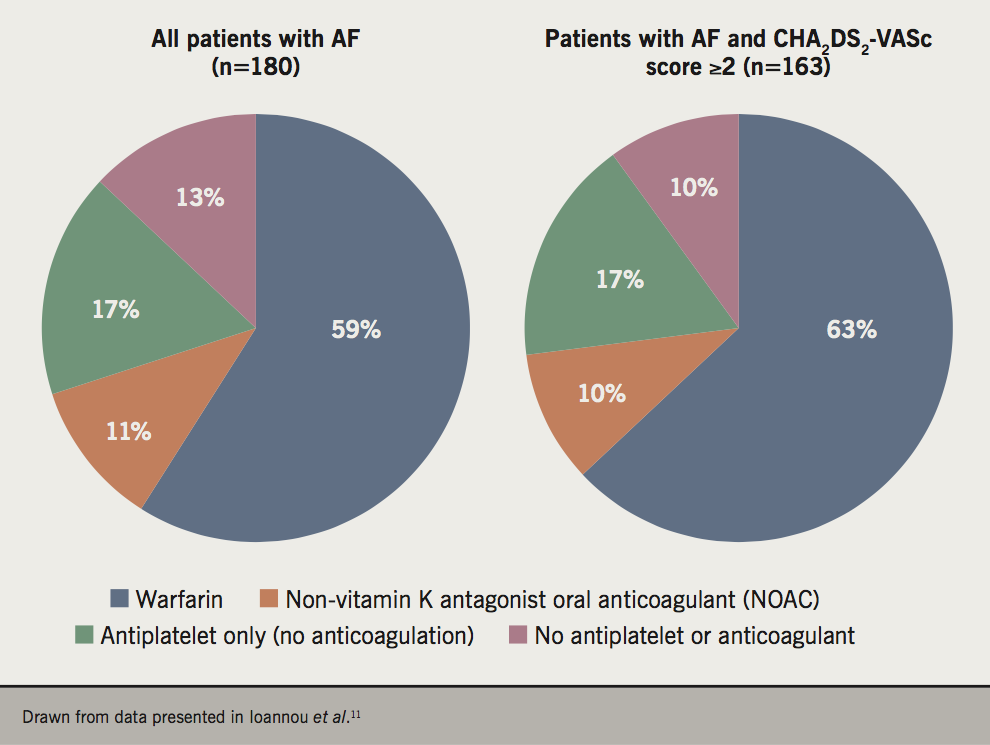
A substantial proportion of people with AF at elevated risk of stroke continue to receive antiplatelet agents, or no therapy, rather than an anticoagulant (figure 1).11 The Quality Outcomes Framework (QoF) in the UK is designed to encourage primary care practices to apply evidence-based care. Using QoF in England as an example, financial incentives are in place to encourage keeping of a local practice registry of patients with AF (QoF Indicator AF001), the use of CHA2DS2-VASc score to evaluate stroke risk in these patients (AF006), and the proportion of patients with AF and CHA2DS2-VASc score ≥2 who receive anticoagulation (AF007).12 However, the current lower thresholds for receiving payment are low, at 40% of patients for both AF006 and AF007. In the author’s opinion, primary care practice should be aiming at much higher concordance with guideline-based care in the management of these high-risk patients.
Safety
NOACs slightly increase the risk of gastrointestinal bleeding versus standard anticoagulation,13 but this is mitigated by generally less serious bleeds compared to warfarin.14 Physicians’ fear of bleeding may lead to under use of anticoagulation in the management of AF.15 Physicians require more education on the potential for NOACs to reduce the risk of serious bleeding, particularly primary-care physicians who usually do not see patients with an intracranial bleed.
It is important to select the right dose of anticoagulation for the individual patient. A study from an administrative claims database in the USA found frequent overdosing of NOACs for people with renal dysfunction requiring a dose reduction (43% of these patients), and these patients were at increased risk of bleeding events without reduction in the risk of stroke, compared with patients not requiring a dose reduction.16 Conversely, in this study, a dose reduction was applied for 13% of patients who did not require it, and these patients were at increased risk of stroke without reduced risk of bleeding. Guidelines need to be clear that patients should receive their individualised, indicated dose when prescribed a NOAC.
Monitoring
Physicians may worry that there is no way to tell if a NOAC is working, having been accustomed to regular international normalised ratio (INR) tests for their patients on warfarin, although others appreciate the reduced monitoring burden with NOACs versus warfarin.17 Some patients also feel comforted to have ‘a number’ to tell them that their medication is working, and may feel secure in their regimen of regular testing.17 The lack of a treatment to reverse the effect of a NOAC may also be a concern, although reversal agents are available or in development for use in patients receiving a NOAC (Factor Xa antagonists or direct thrombin inhibitors) who present with a major bleeding episode.18 Time will tell as to how often these new reversal agents are required.
Time in therapeutic range (TTR) is measured as an indicator of adherence to vitamin K antagonist (VKA) anticoagulant therapy. Guidance from the National Institute for Health and Care Excellence (NICE) specifies suboptimal compliance as TTR <65%. Median TTR was 68.4% in ENGAGE-AF TIMI-481 and 63.5% in Hokusai-VTE,2 illustrating the difficulty of maintaining adequate anticoagulation on warfarin even in the structured environment of a randomised-controlled trial. A recent report from the UK showed that more than one-third of warfarin-treated patients with AF managed in primary care had TTR <65%.11
Cost-effectiveness
NOACs are currently branded medications, and hence are expensive. Nevertheless, NOACs, including edoxaban, have been found to be cost-effective compared with warfarin in populations with AF or at risk of recurrent VTE.19-23 Such evaluations tend not to include the costs of the regular INR measurement visits required to support warfarin treatment, which place a substantial burden on local healthcare systems; these costs should be included to provide a genuine comparison of overall treatment strategies. A rigorous and comprehensive assessment of the cost-effectiveness of warfarin in comparison with NOACs in the primary-care sector has yet to be conducted.
Patient subgroups
Patients with prior stroke or transient ischaemic attack (TIA) are at markedly elevated risk of future cerebrovascular disease and at higher risk of bleeding events, an issue which remains under appreciated in the primary-care setting. Prescribing of NOACs varies greatly between Clinical Commissioning Groups in the UK,24 which may impose barriers to prescription of a NOAC for a patient who is naïve to a VKA, such as a trial of warfarin, or failing a genetic test for warfarin sensitivity. This would seem unnecessary for most patients, when clinical trials have demonstrated the comparable efficacy and reduced bleeding risk of NOACs versus warfarin, as described above.
Edoxaban is administered once daily (following ≥5 days of parenteral anticoagulation in VTE indications), in comparison with twice-daily administration for some other NOACs,25 which may be an advantage in terms of convenience, especially for active adult patients, and in terms of reduced potential for polypharmacy for patients with significant comorbidity. The trend to increased efficacy of edoxaban with increasing age in preventing recurrent VTE in Hokusai-VTE is of interest in the management of elderly patients with VTE.26
Edoxaban should not be administered to patients with end-stage renal disease (on dialysis or with creatinine clearance [CrCl] <15 ml/min).27 Renal function close to this level would be a concern during the use of edoxaban, in case an intercurrent illness caused an acute reduction in renal function to below this cut-off. The ENGAGE-AF TIMI-48 and Hokusai-VTE trials, on which the approval of edoxaban was based, excluded patients with CrCl <30 ml/min at baseline, and patients whose CrCl declined below this level during the study were retained within the study for analysis.1,2 An apparent non-statistically significant diminution of efficacy was only observed for edoxaban in ENGAGE-AF TIMI-48 for patients with a high level of CrCl (>95 ml/min), which would be encountered infrequently in the primary-care setting. Overall, edoxaban appears to have an adequate margin for renal safety.
Many patients with reduced renal function are elderly; patients at risk of falling, a challenging and also usually older group for anticoagulation, derived a larger absolute benefit for reduced bleeding complications with edoxaban versus warfarin in ENGAGE-AF TIMI-48.28 This was an interesting finding, as such patients can end up receiving aspirin rather than anticoagulation, due to their higher risk of bleeding.
Heart failure is another common comorbidity, and AF co-presents frequently with heart failure with preserved left ventricular ejection fraction (HFpEF).29 Anticoagulation is challenging in this population, but remains the only management strategy that has significantly improved outcomes in this population to date.29 Importantly, the efficacy of edoxaban in preventing stroke or systemic embolic events was not diminished in people with HFpEF.30 Registry data from the USA suggest that anticoagulant therapy appears to be underused in patients with HFpEF and AF, suggesting a lack of appreciation of the adverse impact on prognosis in this combination of conditions.31
Conclusions
The evidence-based application of NOACs has the potential to contribute to optimised, individualised stroke prophylaxis in a broad range of patients with AF, and in the treatment of VTE and prevention of recurrent VTE. Primary care physicians may need education on the role and appropriate use of these agents.
Key messages
- Non-vitamin K antagonist oral anticoagulants (NOACs) are suitable for stroke prophylaxis, venous thromboembolism (VTE) treatment and prevention of recurrent VTE for a broad range of subgroups of patients managed in the primary-care setting
- Once-daily anticoagulation with edoxaban (following ≥5 days of parenteral anticoagulant in VTE indications) may offer convenient delivery of NOAC-based therapy, compared with the twice-daily administration of some other NOACs and the complex, burdensome regimen of warfarin
- Nevertheless, NOACs remain underused in routine practice and are frequently underdosed, and education on their use may benefit primary-care physicians and patients
Conflicts of interest
NR has been a speaker and advisor for Bayer and speaker for Boehringer-Ingelheim; Member of Faculty of BMS/Pfizer Masterclass. Previously NHS England Clinical Champion for AF and AHSN NENC AF project advisor; now recently retired from general practice.
Acknowledgement
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial assistance, funded by the British Journal of Cardiology.
Nigel Rowell
Retired General Practitioner, previously at the Endeavour Practice, Middlesbrough, UK
Email: n.rowell@btinternet.com
Articles in this supplement
Introduction
1. Insights in patients with atrial fibrillation and co-existing cardiovascular disease
2. Insights in stroke prevention in patients with a prior history of stroke or transient ischaemic attack
3. Insights in the treatment and prophylaxis of venous thromboembolism
References
1. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. https://doi.org/10.1056/NEJMoa1310907
2. Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. https://doi.org/10.1056/NEJMoa1306638
3. Larsen TB, Nielsen PB, Skjøth F, Rasmussen LH, Lip GY. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: a semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS One 2014;9:e114445. https://doi.org/10.1371/journal.pone.0114445
4. Sardar P, Chatterjee S, Lavie CJ et al. Risk of major bleeding in different indications for new oral anticoagulants: insights from a meta-analysis of approved dosages from 50 randomized trials. Int J Cardiol 2015;179:279–87. https://doi.org/10.1016/j.ijcard.2014.11.101
5. Senoo K, Lau YC, Dzeshka M, Lane D, Okumura K, Lip GY. Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation – meta-analysis. Circ J 2015;79:339–45. https://doi.org/10.1253/circj.CJ-14-1042
6. Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2015;262:516–22. https://doi.org/10.1007/s00415-014-7462-0
7. Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism – a systematic review with indirect comparisons. Vasa 2014;43:353–64. https://doi.org/10.1024/0301-1526/a000373
8. Providência R, Grove EL, Husted S, Barra S, Boveda S, Morais J. A meta-analysis of phase III randomized controlled trials with novel oral anticoagulants in atrial fibrillation: comparisons between direct thrombin inhibitors vs. factor Xa inhibitors and different dosing regimens. Thromb Res 2014;134:1253–64. https://doi.org/10.1016/j.thromres.2014.10.002
9. Mant J, Hobbs FDR, Fletcher K et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trail. Lancet 2007;370:493–503. https://doi.org.10.1016/S0140-6736(07)61233-1
10. Connolly SJ, Eikelboom J, Joyner C et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. https://doi.org/10.1056/NEJMoa1007432
11. Ioannou A, Metaxa S, Kassianos G, Missouris CG. Anticoagulation for the prevention of stroke in non-valvular AF in general practice: room for improvement. Drugs Context 2016;5:212295. https://doi.org/10.7573/dic.212295
12. NHS England. Summary of changes to QoF 2018/19 – England. Available at: https://www.nhsemployers.org/-/media/Employers/Documents/Primary-care-contracts/QOF/2018-19/201819-Quality-and-outcomes-framework-summary-of-changes.pdf [accessed March 2019].
13. Cheung KS, Leung WK. Gastrointestinal bleeding in patients on novel oral anticoagulants: Risk, prevention and management. World J Gastroenterol 2017;23:1954–63. https://doi.org/10.3748/wjg.v23.i11.1954
14. Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:P955–62. https://doi.org/10.1016/S0140-6736(13)62343-0
15. Sen S, Dahlberg KW. Physician’s fear of anticoagulant therapy in nonvalvular atrial fibrillation. Am J Med Sci 2014;348:513–21. https://doi.org/10.1097/MAJ.0000000000000349
16. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017;69:2779–90. https://doi.org/10.1016/j.jacc.2017.03.600
17. Canadian Agency for Drugs and Technologies in Health. Warfarin management in patients with atrial fibrillation – current practice study. CADTH Optimal Use Report, No. 1.2D2012. Ottawa (ON): CADTH. Available at: https://www.ncbi.nlm.nih.gov/books/NBK361753/ [accessed March 2019].
18. Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol 2018;15:273–81. https://doi.org/10.1038/nrcardio.2017.223
19. Vilain KA, Yang MC, Hui Tan E et al. Cost-effectiveness of edoxaban vs. warfarin in patients with atrial fibrillation based on results of the ENGAGE AF – TIMI 48 trial: Taiwanese perspective. Value Health Reg Issues 2017;12:74–83. https://doi.org/10.1016/j.vhri.2017.03.011
20. Clay E, Jamotte A, Verhamme P et al. Cost-effectiveness of edoxaban compared to warfarin for the treatment and secondary prevention of venous thromboembolism in the UK. J Mark Access Health Policy 2018;6:1495974. https://doi.org/10.1080/20016689.2018.1495974
21. Mendoza-Sanchez J, Silva F, Rangel L et al. Benefit, risk and cost of new oral anticoagulants and warfarin in atrial fibrillation: a multicriteria decision analysis. PLoS One 2018;13:e0196361. https://doi.org/10.1371/journal.pone.0196361
22. Pinyol C, Cepeda JM, Roldan I, Roldan V, Jimenez S, Gonzalez P, Soto J. A systematic literature review on the cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation. Cardiol Ther 2016;5:171–86. https://doi.org/10.1007/s40119-016-0066-2
23. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676–81. https://doi.org/10.1161/STROKEAHA.111.000402
24. Millett D. NOAC prescribing varies 16-fold between CCG areas. GPOnline. Available at: https://www.gponline.com/noac-prescribing-varies-16-fold-ccg-areas/cv-thromboembolic-disorders/atrial-fibrillation/article/1394082 [accessed March 2019].
25. National Institute for Health and Care Excellence. Anticoagulation – oral. London: NICE, 2017. Available from: https://cks.nice.org.uk/anticoagulation-oral
26. Kato ET, Giugliano RP, Ruff CT et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. JAMA 2016;5:e003432. https://doi.org/10.1161/JAHA.116.003432
27. Daiichi Sankyo UK Limited. European summary of product characteristics. Lixiana 60 mg film-coated tablets. Available from: https://www.medicines.org.uk/emc/product/6905/smpc [accessed March 2019].
28. Steffel J, Giugliano RP, Braunwald E et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol 2016;68:1169–78. https://doi.org/10.1016/j.jacc.2016.06.034
29. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016;68:2217–28. https://doi.org/10.1016/j.jacc.2016.08.048
30. Magnani G, Giugliano RP1, Ruff CT et al. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail 2016;18:1153–61. https://doi.org/10.1002/ejhf.595
31. Contreras JP, Hong KN, Castillo J et al. Anticoagulation in patients with atrial fibrillation and heart failure: insights from the NCDR PINNACLE-AF registry. Clin Cardiol 2019;42:339–45. https://doi.org/10.1002/clc.23142



