A wide range of medications including antimalarial preparations (chloroquine, hydroxychloroquine), macrolide antibiotics (azithromycin) and the interleukin-6 inhibitor (tocilizumab) may be effective in treating patients with coronavirus disease 2019 (COVID-19). Such agents may be associated with cardiotoxicity, and the purpose of this brief review is to draw attention to potential areas of pharmacovigilance. These include prolongation of the QT-interval and the development of occult cardiomyopathy. Alternatively, some of the agents seem to have minimal impact on the cardiovascular system. The review highlights the need for an ongoing evaluation of such agents within carefully constructed clinical trials with embedded attention to cardiovascular safety.
The reason to be cautious when evaluating curative or symptomatic treatments is the fact that SARS-CoV-2 has affected large segments of the population, with disproportionate mortality rates within certain subgroups. Some of the enhanced mortality may reflect inherent cardiovascular disease risk factors related to acute COVID-19 infection.
It is hoped that the review will stimulate a greater awareness of potential cardiovascular side effects and encourage reporting of those in future trials.
Introduction

SARS-CoV-2 has rapidly become a worldwide health emergency. The declaration by the World Health Organisation (WHO) in March 2020 of a global pandemic has underscored the widespread morbidity and mortality caused by the virus. Concerted efforts by healthcare and research communities are ongoing to establish the efficacy and potency of various pharmacotherapeutics. It has been shown that coronavirus disease 2019 (COVID-19) affects multiple organs and has heterogeneous effects on the cardiovascular system. This is also accompanied by enhanced morbidity and mortality in patients with pre-existing cardiovascular disease.1 In urgent evaluation of established drugs for a novel indication, healthcare practitioners should be vigilant to potential risks of cardiotoxicity and iatrogenic harm.
COVID-19 trial drugs
A large number of clinical trials have been commenced with particular focus on chloroquine (hydroxychloroquine, chloroquine), interleukin-6 (IL-6) receptor antagonists (tocilizumab), retroviral protease inhibitors (lopinavir), and RNA polymerase inhibitors (remdesivir).
Chloroquine (CQ) and hydroxychloroquine (HCQ)
The initially promising results of small trials of CQ and HCQ in patients with COVID-19 were not reproduced. In contrast a paper published in the Lancet, which was subsequently retracted, raised concerns that CQ/HCQ was seemingly associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19 in a multi-national registry analysis.2 A subsequent press release on 5 June 2020 of preliminary results from the Randomised Evaluation of COVid-19 thERapY (RECOVERY) trial stated that there is no clinical benefit from the use of HCQ in hospitalised patients with COVID-19, peer-reviewed data are awaited.3 A randomised, double-blind, placebo-controlled trial found that HCQ is not effective in the prevention of COVID-19 when used as post-exposure prophylaxis in asymptomatic participants. It was noted that adherence to HCQ was moderate with 40% reporting a side effect, most commonly nausea, loose stools, and abdominal discomfort. However, no serious adverse reactions or cardiac arrhythmias were reported.4
Concerns have been raised due to increased risks of cardiotoxicity, particularly related to corrected QT-interval (QTc) prolongation.5,6 The risk of QTc prolongation increases with use of higher doses of HCQ/CQ, presence of renal dysfunction, electrolyte abnormalities and concomitant use of other QTc prolonging agents (e.g. azithromycin and/or selective serotonin re-uptake inhibitors).6,7 The risk of undetected QTc prolongation can be worsened by a rationed usage of cardiac monitoring and limited use of electrocardiography (ECG) due to concerns about staff exposure to infected patients.
The reported rate of QTc prolongation caused by CQ/HCQ alone or in combination with azithromycin used in the treatment of patients with COVID-19 has varied, and most studies assessed the drugs’ benefits rather than cardiovascular complications. Although prolonged QTc, with QTc of ≥500 ms or change in QTc of ≥60 ms, during treatment with CQ/HCQ alone or in combination with azithromycin was observed in between 8.6% and 93%,6-8 there are very limited reports on a significant risk of sudden death or torsades de pointes. However, it is important to remain vigilant for a potential pro-arrhythmogenic effect of CQ/HCQ, particularly in patients with prolonged QT-interval, electrolyte disturbance and those with conduction abnormalities. Another potential cardiac complication of chloroquines is the occult development of HCQ/CQ-induced cardiomyopathy. Though uncommonly reported in currently available publications,9-11 progressive left ventricular dysfunction may be one of many causes of clinical deterioration in patients with COVID-19. Cardiac arrhythmia or reduced left ventricular function due to chloroquines should be considered in patients with worsening cardio/respiratory symptoms/status, and cardiac monitoring should be facilitated to avoid iatrogenic morbidity and mortality.
IL-6 receptor inhibitor: tocilizumab
SARS-CoV-2 infection can result in cytokine storm, endothelial dysfunction and inappropriate activation of the clotting system. Following its clinical use for cytokine release syndromes in oncology, the IL-6 receptor inhibitor tocilizumab has been appraised in multiple retrospective studies and reviews. Until recently there has been heterogeneous data representing an unselected real-world population, ranging from ward patients to critically ill intensive care patients, with various control groups receiving a wide range of standard care and other trial drugs, as well as comparison to historical control groups. However, one randomised, double-blind, placebo-controlled, multi-centre phase III study evaluated the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA). It did not meet its primary end point of improved clinical status or the key secondary end point of reduced patient mortality.12 A randomised, double-blind, multi-centre study to evaluate the efficacy and safety of remdesivir plus tocilizumab compared with remdesivir plus placebo in hospitalised participants with severe COVID-19 pneumonia (REMDACTA) is currently recruiting.13
So far there are no data on adverse cardiovascular effects. However, a large-scale real-life pharmacovigilance assessment found increased reporting of hepatic, pancreatic and pulmonary reactions (e.g. interstitial pneumonitis and alveolar damage).14 This highlights the importance of monitoring patients for unpredictable adverse events in clinical trials and off-label use of tocilizumab.
Antiviral drugs
The focus of attention has been split between protease inhibitors, such as ritonavir and darunavir, and the RNA polymerase inhibitor, remdesivir. Protease inhibitors are well established in the treatment of patients with human immunodeficiency virus (HIV). A single-centre, open-label, randomised-controlled trial (RCT) by Cao et al. concluded that the administration of lopinavir–ritonavir was not associated with clinical improvement or mortality in seriously ill patients with COVID-19.15 However, concerns were raised about the statistically underpowered trial resulting in an inability to show this outcome.16 The results of the WHO SOLIDARITY trial, including lopinavir–ritonavir alone or in combination with interferon-beta for patients with COVID-19, are awaited. Protease inhibitors have been associated with adverse cardiovascular outcomes in patients with HIV.17 However, clinicians should be aware that the data accumulated within the sphere of HIV medicine reflect long-term usage of such drugs. The short-term cardiovascular effects are unknown. Notably, protease inhibitors can exert effects on the cytochrome system (CYP), specifically the CYP3A4 isoenzyme. With varying effects including inhibition, this may have clinical relevance on co-prescribed medications including statins, anti-arrhythmic drugs (amiodarone), diltiazem, antifungals and macrolide antibiotics.
Remdesivir is another antiviral agent, which has garnered scientific and general media attention. It is a nucleoside analogue that inhibits RNA polymerase and, hence, interferes with viral replication, which leads to a decrease in circulating viral RNA. It has previously been studied in the treatment of Ebola virus disease.18 Initial results in the evaluation of remdesivir in patients with COVID-19 were inconclusive.19 The main encouraging data originated from the double-blind placebo-controlled study conducted by the National Institute of Allergy and Infectious Diseases, USA, including over 1,000 patients.20 The study was stopped early due to positive efficacy of remdesivir, resulting in emergency Food and Drug Administration (FDA) approval of remdesivir. Adverse events data are not fully available to establish the direct correlation between remdesivir and the observed events. Nevertheless, this agent was the first licensed in the UK for the treatment of SARS-CoV-2.21
The signal from these studies has encouraged global consideration of remdesivir, but many questions remain about its applicability within the treatment of COVID-19. The exact timing of using the drug and the duration of treatment are unknown, initial data indicate that remdesivir may be associated with hypotension.22
To date there are no data from large randomised trials available to inform us about the reduction of viral load in relation to remdesivir use, as well as reduction in mortality. A study on compassionate use of remdesivir reported a clinical improvement of 68% in a patient group selected by Gilead Sciences, upon request by clinicians across nine different countries. Although encouraging, these data are too heterogeneous to be extrapolated and larger RCTs are required to inform decisions on use of remdesivir in wider practice. Safety data on remdesivir are lacking, and although atrial fibrillation and hypotension were reported as adverse events,23 further information is needed.
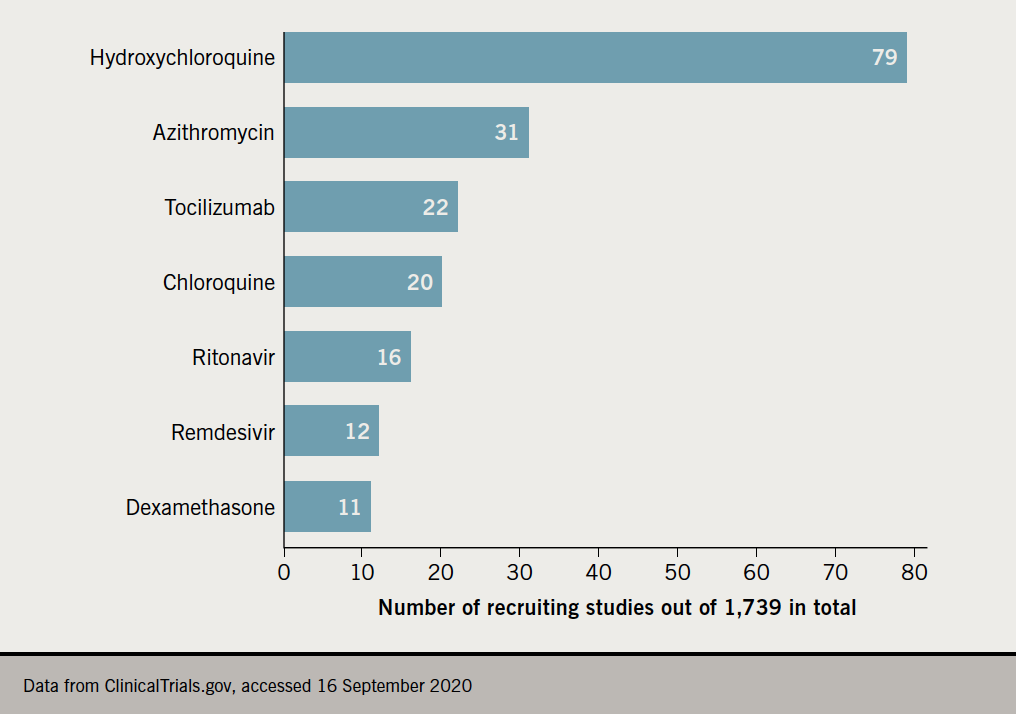
Summary of mechanism of action, cardiotoxic effects and current trials on treatment for COVID-19 are shown in table 1 and figure 1.
Table 1. Summary of mechanisms of action and cardiotoxic effects of selected drugs evaluated in coronavirus disease 2019 (COVID-19)
| Name | Mechanism of action | Toxicity | |
|---|---|---|---|
| Chloroquine/hydroxychloroquine (CQ/HCQ) | General biochemical/cellular, antiviral and immunomodulatory effects25,26 | QTc prolongation (exacerbated by azithromycin)5-8 CQ/HCQ induced cardiomyopathy9-11 |
|
| Tocilizumab | Anti-interleukin-6 receptor monoclonal antibody, reduction of the cytokine’s pro-inflammatory activity and systemic toxicity in cytokine storm | No published evidence of cardiovascular side effects Hepatic, pancreatic and pulmonary reactions like interstitial pneumonitis and alveolar damage14 |
|
| Lopinavir, darunavir | Antiviral, protease inhibitor (PI) used for patients with HIV | Adverse effect on serum triglycerides, lipoprotein A17 | |
| Ritonavir | Cytochrome P450 (CYP)-3A4 inhibitor used in combination with lopinavir and darunavir to increase plasma levels | Interaction with other medications via cytochrome enzymes | |
| Lopinavir–ritonavir | Cardiotoxicity: QT-prolongation Drug interactions due to CYP3A inhibition27 |
||
| Remdesivir | Nucleoside analogue, inhibits RNA polymerase, inhibits viral replication by the mechanism of a decrease in circulating viral RNA | Arrhythmia (atrial fibrillation) Hypotension Cardiopulmonary failure Cardiac arrest22,23 |
Conclusion
SARS-CoV-2 has rapidly become a global health emergency and multi-national work is ongoing to discover and harness novel therapeutics. However, careful application of science and large adequately powered RCTs are needed to ensure safe use of proposed treatments. A preliminary report from the UK RECOVERY trial, with treatment arms including low-dose dexamethasone, azithromycin, tocilizumab and convalescent plasma, demonstrated that dexamethasone lowered 28-day mortality in patients with COVID-19 receiving either invasive mechanical ventilation or oxygen alone at randomisation. However, the possibility of harm among patients who did not require oxygen was highlighted, without further specification of adverse events.24 Peer-reviewed results from the remaining treatment arms of the RECOVERY trial, as well as the COVACTA and REMDACTA trials investigating tocilizumab, and the WHO SOLIDARITY trial, including lopinavir–ritonavir alone or in combination with interferon-beta, are awaited. Consideration of safety and adverse events should be reported alongside any positive findings. Finally, when using novel or unfamiliar drugs in the context of COVID-19, it is important that healthcare professionals are aware of the potential risks of cardiotoxicity and pay particular attention to the cardiovascular background history of their patients.
Key messages
- Cardiovascular effects of coronavirus disease 2019 (COVID-19) medical therapies are poorly understood
- Chloroquine and hydroxychloroquine may lead to QTc prolongation and occult cardiomyopathy
- Clinical trials on COVID-19 therapies should incorporate vigorous safety monitoring
Conflicts of interest
None declared.
Funding
None.
References
1. Du RH, Liang LR, Yang CQ et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J 2020;55:2000524. https://doi.org/10.1183/13993003.00524-2020
2. Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 2020;[online]. https://doi.org/10.1016/S0140-6736(20)31180-6
3. Horby P, Mafham M, Linsell L et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv 2020 [preprint]. https://doi.org/10.1101/2020.07.15.20151852
4. Boulware DR, Pullen MF, Bangdiwala AS et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517–25. https://doi.org/10.1056/NEJMoa2016638
5. Borba MG, Val FF, Sampaio VS et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3:e208857. https://doi.org/10.1001/jamanetworkopen.2020.8857
6. Bessière F, Roccia H, Delinière A et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020;5:1067–69. https://doi.org/10.1001/jamacardio.2020.1787
7. Mercuro NJ, Yen CF, Shim DJ et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1036–41. https://doi.org/10.1001/jamacardio.2020.1834
8. Saleh M, Gabriels J, Chang D et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol 2020;13:e008662. https://doi.org/10.1161/CIRCEP.120.008662
9. Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 2018;41:919–31. https://doi.org/10.1007/s40264-018-0689-4
10. Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77–83. https://doi.org/10.1177/2048872612471215
11. Vereckei A, Fazakas Á, Baló T, Fekete B, Molnár MJ, Karádi I. Chloroquine cardiotoxicity mimicking connective tissue disease heart involvement. Immunopharmacol Immunotoxicol 2013;35:304–06. https://doi.org/10.3109/08923973.2013.766801
12. Roche. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29, 2020. Available at: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm
13. Clinicaltrials.gov. A study to evaluate the efficacy and safety of remdesivir plus tocilizumab compared with remdesivir plus placebo in hospitalized participants with severe COVID-19 pneumonia (REMDACTA). Available at: https://clinicaltrials.gov/ct2/show/NCT04409262
14. Gatti M, Fusaroli M, Caraceni P, Poluzzi E, De Ponti F, Raschi E. Serious adverse events with tocilizumab: pharmacovigilance as an aid to prioritize monitoring in COVID-19. Br J Clin Pharmacol 2020;[online first]. https://doi.org/10.1111/bcp.14459
15. Cao B, Wang Y, Wen D et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. https://doi.org/10.1056/NEJMoa2001282
16. Meini S, Pagotto A, Longo B, Vendramin I, Pecori D, Tascini C. Role of lopinavir/ritonavir in the treatment of Covid-19: a review of current evidence, guideline recommendations, and perspectives. J Clin Med 2020;9:2050. https://doi.org/10.3390/jcm9072050
17. Périard D, Telenti A, Sudre P et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation 1999;100:700–05. https://doi.org/10.1161/01.CIR.100.7.700
18. Mulangu S, Dodd LE, Davey Jr RT et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293–303. https://doi.org/10.1056/NEJMoa1910993
19. Wang Y, Zhang D, Du G et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–78. https://doi.org/10.1016/S0140-6736(20)31022-9
20. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med 2020;383:994. https://doi.org/10.1056/NEJMc2022236
21. Kmietowicz Z. Covid-19: selected NHS patients will be treated with remdesivir. BMJ 2020;369:m2097. https://doi.org/10.1136/bmj.m2097
22. Goldman JD, Lye DC, Hui DS et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020;383:1827–37. https://doi.org/10.1056/NEJMoa2015301
23. Grein J, Ohmagari N, Shin D et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020;382:2327–36. https://doi.org/10.1056/NEJMc2015312
24. Horby P, Lim WS, Emberson JR et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med 2020;[online first]. https://doi.org/10.1056/NEJMoa2021436
25. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect Dis 2003;3:722–7. https://doi.org/10.1016/S1473-3099(03)00806-5
26. Tripathy S, Dassarma B, Roy S, Chabalala H, Matsabisa MG. A review on possible modes of actions of chloroquine/hydroxychloroquine: repurposing against SAR-COV-2 (COVID 19) pandemic. Int J Antimicrob Agents 2020;56:106028. https://doi.org/10.1016/j.ijantimicag.2020.106028
27. Anson BD, Weaver JGR, Ackerman MJ et al. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005;365:682–6. https://doi.org/10.1016/S0140-6736(05)17950-1
