Takotsubo syndrome – also known as broken-heart syndrome, Takotsubo cardiomyopathy, and stress-induced cardiomyopathy – is a recently discovered acute cardiac disease first described in Japan in 1991. This review aims to update understanding on the epidemiology, pathophysiology, clinical presentation, diagnosis, and treatment of Takotsubo syndrome, highlighting aspects of interest to cardiologists and general practitioners.
Background
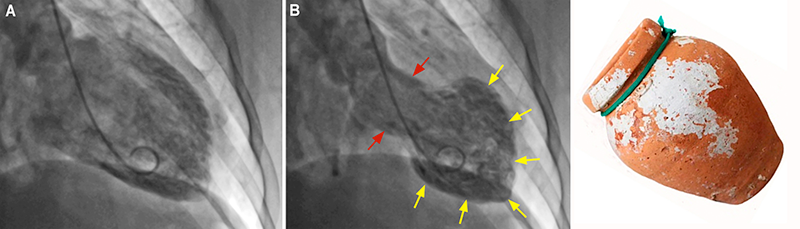
Takotsubo syndrome (TTS) – also known as broken-heart syndrome, Takotsubo cardiomyopathy, and stress-induced cardiomyopathy – is a recently discovered acute cardiac disease first described in Japan in 1991.1 TTS has a clinical presentation with chest pain, ischaemic electrocardiographic (ECG) changes, and elevation of biomarkers, such as cardiac troponin and brain natriuretic peptide (BNP), triggered by significant emotional or physical stress, and accompanied by distinct patterns of transient left ventricular dysfunction.2,3 This contractile dysfunction classically adopts an apical ballooning shape of the left cardiac chamber resembling a Japanese octopus trap named a Takotsubo (figure 1).4,5 TTS mimics acute coronary syndrome (ACS) and can be indistinguishable from that disease if the coronary arteries are demonstrated to be normal.6,7 In this scenario, a differential diagnosis – although challenging – is essential for ensuring correct treatment.
Epidemiology
TTS is more frequent in women than men; thus, about 90% of patients are postmenopausal women.4,8 Approximately 2% of all patients presenting with suspected ACS also have TTS.9-11
Aetiology, predisposition, risk factors, and triggers
The aetiology of TTS is not fully understood, despite intensive research into its possible causes.
Endocrine factors
The higher incidence of TTS in postmenopausal women suggests hormonal influences. Reduced oestrogen levels during menopause increase endothelial dysfunction (imbalance between vasoconstricting and vasodilating factors) leading to microvascular coronary artery spasm, one of the pathogenetic mechanisms proposed for TTS.12
Genetics
A few studies have reported an association between TTS and the expression of some genes, such as BAG3 and GRK-5.13,14 However, there are conflicting results.15 Currently, there is a large genetic study ongoing among patients with TTS to identify potential genetic predisposition to the disease.16
Psychogenic factors
There is a higher prevalence of TTS in patients with neurological and psychiatric pathologies.3 This association could represent an extracardiac association that should be considered. Depression and anxiety are more common in patients with TTS than in those with ACS or in healthy controls.17,18 Depressed patients have an exaggerated norepinephrine response to emotional stress,19 and patients with anxiety have decreased re-absorption of norepinephrine because of failure in re-uptake transporters.20 TTS can also occur in patients with central nervous system diseases involving brain–heart interactions,21 such as ischaemic stroke,22 subarachnoid haemorrhage,23 and seizures.24
Triggers
Emotional and physical stressors include one or more of the following: divorce, illness or death of a loved one, job loss, bad financial news, earthquakes and aftershocks, car accidents, strokes, seizures, asthma attacks, phaeochromocytoma, giving birth, cancers, infectious diseases, surgery, and anaesthesia.4 It has even been triggered by SARS-CoV-2 infection.25
Pathophysiology
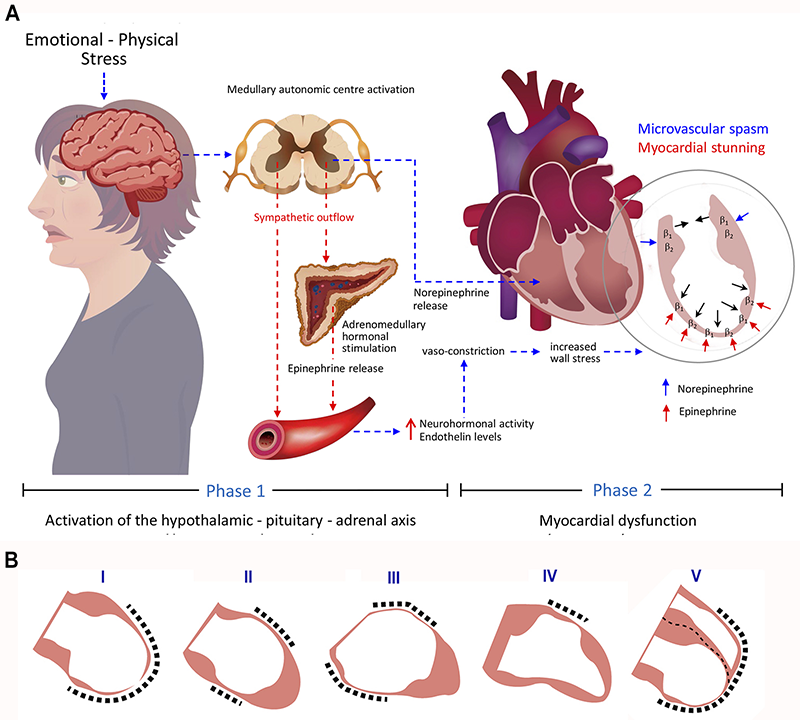
The exact pathophysiology of TTS remains incompletely understood. Nevertheless, the hypothesis of the involvement of a catecholamine surge is the most accepted, currently. This involves an adrenergic cascade through the hypothalamic–pituitary–adrenal axis.26,27 This pathogenic mechanism involves two phases, shown in figure 2A. Phase 1 involves activation of the hypothalamic–pituitary–adrenal axis, and phase 2 involves myocardial dysfunction induced by sympathetic hyperactivity. The first phase begins after an acute psychological and/or physical stress resulting in increased circulating and myocardial catecholamine levels.28,29 During the second phase, different mechanisms have been considered to explain the cardiotoxicity of catecholamines producing myocardial dysfunction. Among these, the most prominent are direct catecholamine-mediated myocardial stunning and microvascular spasm.27
Myocardial stunning
In this, rising catecholamine levels act on beta-adrenergic receptors, triggering the onset of myocardial dysfunction and affecting inotropic cardiac function.28 During severe stress, circulating epinephrine peaks, making the beta-adrenergic receptors most sensitive to its negative inotropic effects.26 Beta-adrenergic receptors have their highest concentration in the apical myocardium, with a gradient decrease from apex to base, which could explain the left ventricular dysfunction pattern most typically seen in patients with TTS (figure 2A).30 The causes of the atypical and different anatomical variants in TTS are unknown. The affected myocardium observed in cases of TTS is accompanied by severe histological abnormalities resulting from the direct toxicity of catecholamines, as well as catecholamine-mediated microcirculatory disturbances followed by ischaemia, however, with potential for rapid functional recovery.31
As will be discussed later in this review, the lack of persistent myocardial damage in patients with TTS is demonstrated in the findings observed on cardiac magnetic resonance imaging, showing only significant myocardial oedema with no evidence of late gadolinium enhancement. This excludes the possibility of significant fibrosis, suggesting that the damage to the dysfunctional myocardium is transient.32
Microvascular spasm
Since TTS was first described, in addition to spasm of the epicardial coronary arteries, diffuse coronary vasoconstriction was documented and can have a pathogenic role.1 Some authors consider that severe and prolonged microvascular constriction causes acute transient myocardial ischaemia in patients with TTS, leading to a severe impairment of left ventricle (LV) contractility involving areas of increased wall stress, such as the mid-left ventricular wall and the apex.32,34 Therefore, TTS may be considered a novel form of ACS.35
Clinical presentation
TTS usually presents clinically as an ACS, mostly in postmenopausal women, triggered by an episode of acute emotional or unusual physical stress. The most common symptoms are chest pain and dyspnoea.4 Physical examinations can be normal or with nonspecific findings.
According to left ventriculography studies, four anatomical variants of TTS have been described: the apical type (approximately 80%), represents the most commonly described or typical variety characterised by apical ballooning, hypo-, a-, or dyskinesia of mid-apical myocardial segments. This is followed, in terms of frequency, by the atypical variants: the midventricular type (15–18%), featuring hypo-, a-, or dyskinesia of midventricular segments; the basal type (2%) where only basal segments are involved; and the focal type (1.5%) mainly affecting an anterolateral segment.3,36 Other variants include isolated right ventricular dysfunction or biventricular dysfunction (figure 2B).37
Table 1. Clinical predictors for the diagnosis of Takotsubo syndrome
| Criteria | Points |
|---|---|
| Female sex | 25 |
| Emotional trigger | 24 |
| Physical trigger | 13 |
| Absence of ST-segment depression* | 12 |
| Psychiatric disorders | 11 |
| Neurological disorders | 9 |
| QTc prolongation | 6 |
| Total | 100 |
| *Except in lead aVR | |
TTS is clinically indistinguishable from acute myocardial infarction (AMI), so its diagnosis is frequently a challenge. A critical score to differentiate TTS from ACS was proposed by the International Takotsubo Registry. This score includes seven criteria, which are given different points according to their diagnostic importance, as shown in table 1.38 Thus, a patient with a score of 30 points has a predictive probability of <1% of having TTS, and one with >70 points has a probability of approximately 90% of being diagnosed with TTS.38 The diagnosis of TTS is mainly based on proposed guidelines, such as those of the Mayo Clinic,10 the European Society of Cardiology,39 and the international diagnostic criteria (InterTAK Diagnostic Score).4 The latter recommends the use of cardiac magnetic resonance (CMR) imaging to exclude infectious myocarditis and for diagnostic confirmation in patients with wall motion abnormalities extending beyond the myocardial territory of a single coronary artery. Approximately, one-third of patients with suspected TTS can have an apical acute myocardial infarction (AMI), as defined using CMR for the final diagnosis.40 The same recommendation should apply in patients with an old myocardial infarction.41 In this scenario, CMR is essential to differentiate TTS from AMI and myocarditis accurately, demonstrating myocardial oedema and the absence of late gadolinium enhancement, ensuring optimal treatment.42-44
Differentiation from ACS
The diagnosis of ACS relies on the presence of chest pain associated with ECG changes with ischaemic pattern and elevation of circulating levels of biomarkers of myocardial damage (troponin and BNP). On the other hand, TTS usually mimics an ACS and can be indistinguishable from it in patients with suspected ACS and normal coronary arteries on coronary angiography; therefore, an expert committee for TTS has proposed a diagnostic algorithm to help distinguish them.44
Tests used in patients with suspected TTS
ECG
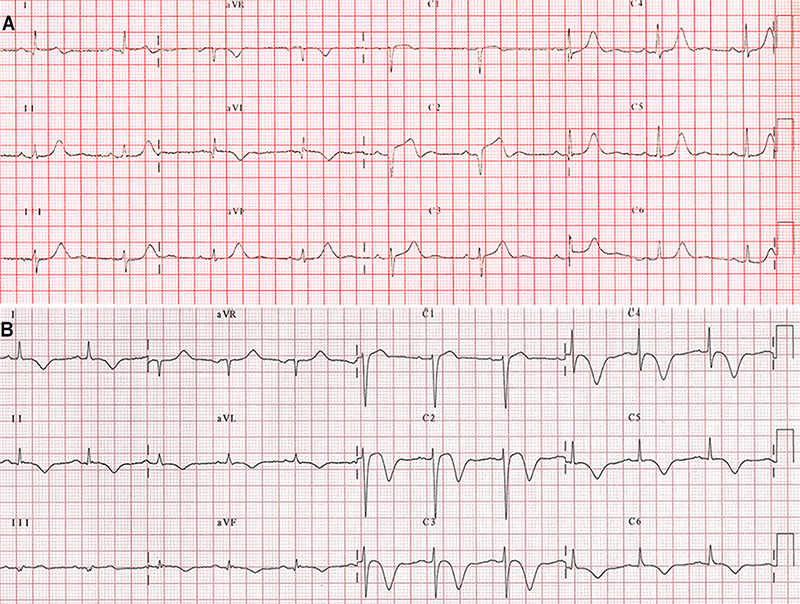
Most patients with TTS have abnormalities on ECG resembling ACS; mainly, ST-segment elevation, T-wave inversion, or both (figure 3).45,46 These abnormalities evolve over time with resolution of ST-segment elevation, when present, and are followed by T-wave inversion and QT-interval prolongation, and a subsequent gradual resolution of T-wave inversion, although it can persist for several months.47
Cardiac biomarkers
On admission to hospital, patients with TTS have elevation of myocardial injury biomarkers, such as creatine kinase–myocardial band isoenzyme (CK–MB), troponin and BNP.44 BNP concentration is higher in patients with TTS compared with those with AMI,3 and early BNP/troponin T, and BNP/CK-MB ratios help to differentiate TTS from AMI with greater accuracy than BNP alone.48 However, CK-MB is only mildly elevated in most patients with TTS.49
Echocardiography
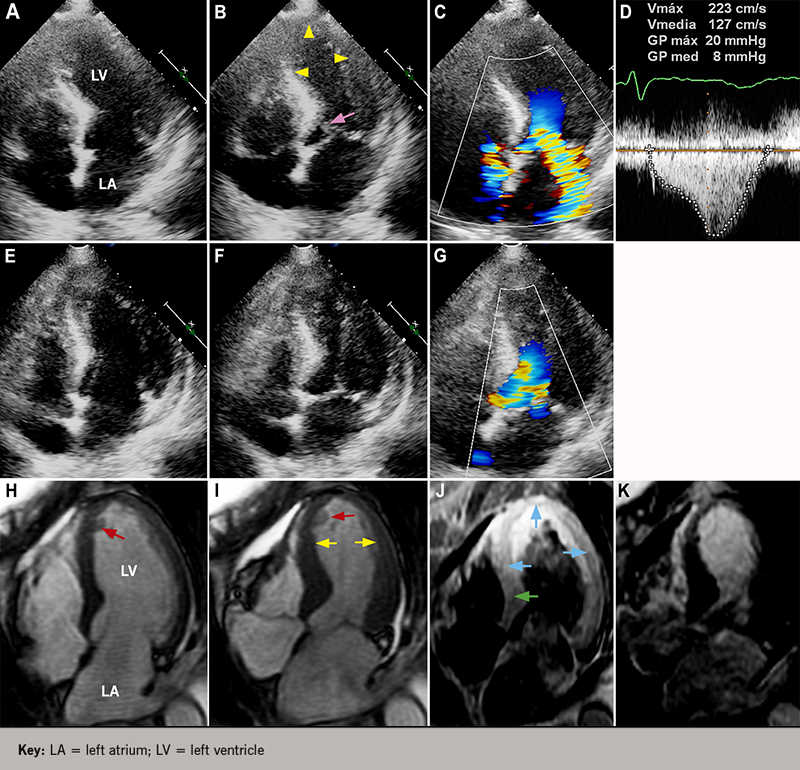
Transthoracic echocardiography (TTE) is a quick, usually available, and very useful non-invasive diagnostic method in the diagnosis of TTS, allowing the visualisation of the regional wall motion abnormalities (RWMA) observed in the typical (figure 4B) and atypical variants of TTS, as well as the global function of the LV. Likewise, it is the diagnostic tool commonly used in following up the resolution of the RWMA of the LV; half of the patients have an early recovery with absence of RWMA within 10 days after the onset of TTS (figure 4F); the other half have a delayed recovery over about two months, and have a higher prevalence of in-hospital complications and higher mortality and should be monitored closely.50 TTE also allows cardiologists to diagnose involvement of the right ventricle (RV) in patients with TTS by detecting RWMA affecting the free wall and/or apex and the dilation of the RV,51-53 and the detection of complications, such as dynamic LV outflow obstruction (LVOTO) and mitral regurgitation (figures 4C and 4D) caused by systolic anterior motion of the mitral leaflet (figure 4B),54-56 as well as thrombus formation in a dysfunctional LV apex.57,58
Coronary angiography and ventriculography
While electrocardiographic findings, cardiac biomarkers, and TTE are useful in the study of patients with TTS, coronary angiography is essential for the differential diagnosis of TTS from ACS. Most patients with TTS have completely normal coronary arteries, but some might have coronary artery disease that should not be considered as an exclusion criterion for TTS.4,10 Left ventriculography illustrates the characteristic wall motion abnormalities seen in the typical (figure 1B) and atypical forms of TTS.
CMR imaging
CMR is increasingly being used as a diagnostic tool in the evaluation of patients with suspected TTS to establish the diagnosis accurately, because it allows the cardiologist to visualise RWMA precisely (figure 4I), quantify global LV and RV functions, and indicate, not just the presence of myocardial oedema representing reversible myocardial injury (figure 4J), but, most importantly, the absence of delayed gadolinium hyperenhancement (figure 4K), allowing the differentiation of TTS from myocardial infarction and myocarditis diseases in which it is present.59,60 CMR is also vital to confirm the diagnosis of TTS in patients presenting with an ACS and with a history of an old myocardial infarction to ensure optimal treatment, as indicated previously.41 The international diagnostic criteria guidelines have recently recommended the use of CMR to exclude infectious myocarditis and confirm diagnosis of TTS.4
Prognosis
Even though TTS has a benign course, with full recovery within a matter of days to a few weeks, around 22% of patients will experience serious cardiac complications such as an ACS.4 According to their prevalence, they are classified as follows:
- Frequent: acute heart failure (12–45%), LVOTO (10–25%) (figure 4D), mitral regurgitation (14–25%) (figure 4C), or cardiogenic shock (6–20%).
- Moderate: atrial fibrillation (5–15%), LV-thrombus (2–8%) (figures 4H and 4I), cardiac arrest (4–6%), and atrioventricular block (5%).
- Rare: tachyarrhythmia, bradyarrhythmia and torsade de pointes (2–5%), death (1–4.5%), ventricular tachycardia/fibrillation (~3%), and acute ventricular septal defect (<1%).41
Regarding the recurrence of TTS, approximately 4–5% of patients who survive the initial event can have a recurrence from three weeks to as long as 4.7 years.61,62 The annual recurrence rate has been reported to vary between 1 and 3.5%.63,64
Treatment
Because most patients with TTS are hospitalised with a suspicion of ACS, they are initially treated according to the clinical guidelines for this disease,65 until the correct diagnosis of TTS is made.
To date, there are no specific or standardised treatments for patients with TTS based on randomised clinical trials,6 and because this is a temporary heart condition, the aim of treatment is to give supportive therapy and minimise complications. Most patients have an uneventful course requiring limited pharmacological therapies, such as the use of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs),3,63 beta blockers,44 and antiplatelet therapies,66 having a full recovery within a couple of weeks. Oral anticoagulation therapy for three months seems reasonable in patients with LV thrombi.58 Patients also need continuous ECG monitoring for at least 48 hours, because of the risk of serious cardiac arrhythmias, such as torsades de pointes, triggered by prolonged corrected QT-intervals.67,68 Patients complicated with acute pulmonary oedema, cardiogenic shock, and those recovering from cardiac arrest require intensive care.44
Patients with pulmonary oedema should be given diuretics and nitroglycerine intravenously, after excluding LVOTO, present in approximately 20% of patients, avoiding worsening of the pressure gradient. LVOTO can be diagnosed during ventriculography or by continuous-wave Doppler ultrasonography (figure 4D). In patients with hypotension without heart failure, clinicians should consider the administration of intravenous fluids, short-acting beta blockers, and, eventually, an LV assist device, e.g. an Impella pump. On the other hand, patients in cardiogenic shock must receive treatment for primary heart failure with catecholamines if LVOTO is not present, or levosimendan could be an alternative inotrope to catecholamines. Furthermore, because of the risk of intraventricular thrombus formation with risk of embolisation, anticoagulation with subcutaneous or intravenous heparin seems reasonable.44 As a long-term treatment, patients should receive ACE inhibitors or ARBs for at least one year to improve survival, but beta blockers are not beneficial.3
Conclusion
TTS is an acute heart condition typically induced by emotional or physical stress, affecting mainly postmenopausal women. The exact pathophysiology of TTS remains incompletely understood; nonetheless, the hypothesis of involvement of a catecholamine surge is the most accepted, currently, specifically, involving an adrenergic cascade through the hypothalamic–pituitary–adrenal axis. It is characterised by transient RWMA of the LV and is clinically indistinguishable from AMI. In this scenario, CMR is essential to differentiate TTS from ACS and acute myocarditis accurately to ensure optimal management. Pharmacological therapies that have proven to be beneficial include the use of ACE inhibitors, ARBs, and antiplatelet therapies. The annual recurrence rate has been reported as 1–3.5%.
Key messages
- Takotsubo syndrome (TTS) is an acute heart condition typically induced by emotional or physical stress affecting mainly postmenopausal women and is clinically indistinguishable from acute myocardial infarction (AMI)
- The exact pathophysiology of TTS remains incompletely understood; nonetheless, the hypothesis of involvement of a catecholamine surge is the most accepted one, currently
- Cardiac magnetic resonance imaging is essential to accurately differentiate TTS from AMI and acute myocarditis, in order to ensure optimal management
- Pharmacological therapies that have proven to be beneficial include the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and antiplatelet therapies
Conflicts of Interest
None declared.
Funding
None.
Editors’ note
See also the case reports by Sekar et al. on pages 35–6 (https://bjcardio.co.uk/2021/01/lockdown-cardiomyopathy-from-a-covid-19-pandemic-to-a-loneliness-pandemic/) and Matthews et al. pages 37–8 of this issue (https://bjcardio.co.uk/2021/03/takotsubo-syndrome-a-predominantly-female-cv-disorder-from-the-perspective-of-primary-care/).
References
1. Sato TH, Uchida T, Dote K, Ishihara M. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In: Kodama K, Haze K, Hori M, eds. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha Publishing Co., 1990; pp. 56–64.
2. Kurowski V, Kaiser A, von Hof K et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007;132:809–16. https://doi.org/10.1378/chest.07-0608
3. Templin C, Ghadri JR, Diekmann J et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. https://doi.org/10.1056/NEJMoa1406761
4. Ghadri JR, Wittstein IS, Prasad A et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. https://doi.org/10.1093/eurheartj/ehy076
5. Dias A, Nuñez Gil IJ, Santoro F et al. Takotsubo syndrome: state-of-the-art review by an expert panel – Part 1. Cardiovasc Revasc Med 2019;20:70–9. https://doi.org/10.1016/j.carrev.2018.11.015
6. Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017;103:1461–9. https://doi.org/10.1136/heartjnl-2016-309783
7. Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart 2018;104:96–102. https://doi.org/10.1136/heartjnl-2017-311579
8. Schneider B, Athanasiadis A, Stöllberger C et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol 2013;166:584–8. https://doi.org/10.1016/j.ijcard.2011.11.027
9. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT –comparison with acute coronary syndrome. Ann Nucl Med 2003;17:115–22. https://doi.org/10.1007/BF02988449
10. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–17. https://doi.org/10.1016/j.ahj.2007.11.008
11. Prasad A, Dangas G, Srinivasan M et al. Incidence and angiographic characteristics of patients with apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: an analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2014;83:343–8. https://doi.org/10.1002/ccd.23441
12. Naegele M, Flammer AJ, Enseleit F et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int J Cardiol 2016;224:226–30. https://doi.org/10.1016/j.ijcard.2016.09.008
13. Citro R, d’Avenia M, De Marco M et al. Polymorphisms of the antiapoptotic protein bag3 may play a role in the pathogenesis of tako-tsubo cardiomyopathy. Int J Cardiol 2013;168:1663–5. https://doi.org/10.1016/j.ijcard.2013.03.050
14. Spinelli L, Trimarco V, Di Marino S, Marino M, Iaccarino G, Trimarco B. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail 2010;12:13–16. https://doi.org/10.1093/eurjhf/hfp173
15. Mattsson E, Saliba-Gustafsson P, Ehrenborg E, Tornvall P. Lack of genetic susceptibility in takotsubo cardiomyopathy: a case-control study. BMC Med Genet 2018;19:39. https://doi.org/10.1186/s12881-018-0544-6
16. Eitel I, Moeller C, Munz M et al. Genome-wide association study in takotsubo syndrome: preliminary results and future directions. Int J Cardiol 2017;236:335–9. https://doi.org/10.1016/j.ijcard.2017.01.093
17. Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol 2010;55:700–01. https://doi.org/1016/j.jacc.2009.10.031
18. Delmas C, Lairez O, Mulin E et al. Anxiodepressive disorders and chronic psychological stress are associated with Tako-Tsubo cardiomyopathy – new physiopathological hypothesis. Circ J 2013;77:175–80. https://doi.org/10.1253/circj.CJ-12-0759
19. Mausbach BT, Dimsdale JE, Ziegler MG et al. Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer’s caregivers. Psychosom Med 2005;67:638–42. https://doi.org/10.1097/01.psy.0000173312.90148.97
20. Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med 2006;68:8–16. https://doi.org/10.1097/01.psy.0000195872.00987.db
21. Finsterer J, Wahhbi K. CNS disease triggering Takotsubo stress cardiomyopathy. Int J Cardiol 2014;177:322–9. https://doi.org/10.1016/j.ijcard.2014.08.101
22. Scheitz JF, Mochmann HC, Witzenbichler B, Fiebach JB, Audebert HJ, Nolte CH. Takotsubo cardiomyopathy following ischemic stroke: a cause of troponin elevation. J Neurol 2012;259:188–90. https://doi.org/10.1007/s00415-011-6139-1
23. Inamasu J, Ganaha T, Nakae S et al. Therapeutic outcomes for patients with aneurysmal subarachnoid hemorrhage complicated by Takotsubo cardiomyopathy. Acta Neurochir (Wien) 2016;158:885–93. https://doi.org/10.1007/s00701-016-2768-6
24. Ghadri JR, Ruschitzka F, Luscher TF, Templin C. Takotsubo cardiomyopathy: still much more to learn. Heart 2014;100:1804–12. https://doi.org/10.1136/heartjnl-2013-304691
25. Meyer P, Degrauwe S, Delden CV, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J 2020;41:1860. https://doi.org/10.1093/eurheartj/ehaa306
26. Akashi Y, Nef H, Lyon A. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol 2015;12:387–97. https://doi.org/10.1038/nrcardio.2015.39
27. Pellicia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation 2017;135:2426–41. https://doi.org/10.1161/CIRCULATIONAHA.116.027121
28. Wittstein IS, Thiemann DR, Lima JAC et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. https://doi.org/10.1056/NEJMoa043046
29. Kume T, Akasaka T, Kawamoto T et al. Assessment of coronary microcirculation in patients with Takotsubo-like left ventricular dysfunction. Circ J 2005;69:934–9. https://doi.org/10.1253/circj.69.934
30. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy – a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008;5:22–9. https://doi.org/10.1038/ncpcardio1066
31. Nef HM, Möllmann H, Kostin S et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J 2007;28:2456–64. https://doi.org/10.1093/eurheartj/ehl570
32. Testa M, Feola M. Usefulness of myocardial positron emission tomography/nuclear imaging in Takotsubo cardiomyopathy. World J Radiol 2014;6:502–06. https://doi.org/10.4329/wjr.v6.i7.502
33. Vitale C, Rosano GM, Kaski JC. Role of coronary microvascular dysfunction in Takotsubo cardiomyopathy. Circ J 2016;80:299–305. https://doi.org/10.1253/circj.CJ-15-1364
34. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–11. https://doi.org/10.1093/eurheartj/eht513
35. Luscher TF, Templin C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards a new definition. Eur Heart J 2016;37:2816–20. https://doi.org/10.1093/eurheartj/ehw057
36. Ghadri JR , Cammann VL, Napp LC et al.; International Takotsubo Registry. Differences in the clinical profile and outcomes of typical and atypical Takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016;1:335–40. https://doi.org/10.1001/jamacardio.2016.0225
37. Medina de Chazal H, Del Buono MG, Keyser-Marcus L et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955–71. https://doi.org/10.1016/j.jacc.2018.07.072
38. Ghadri JR, Camman VL, Jurisic S et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail 2017;19:1036–42. https://doi.org/10.1002/ejhf.683
39. Lyon AR, Bossone E, Schneider B et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. https://doi.org/10.1002/ejhf.424
40. Gandhi H, Rodriguez JE, Reynolds H. Takotsubo cardiomyopathy versus apical infarction in patients with myocardial infarction and non-obstructive coronary artery disease (MINOCA). J Am Coll Cardiol 2017;69:270. https://doi.org/10.1016/S0735-1097(17)33659-8
41. Díaz-Navarro, Villagran F. Takotsubo cardiomyopathy and coronary artery disease: value of cardiac magnetic resonance imaging for diagnostic confirmation: a case report. Eur Heart J Case Rep 2018;3:yty151. https://doi.org/10.1093/ehjcr/yty151
42. Eitel I, Behrendt F, Schindler K et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J 2008;29:2651–9. https://doi.org/10.1093/eurheartj/ehn433
43. Bratis K. Cardiac magnetic resonance in Takotsubo syndrome. Eur Cardiol 2017;1:58–62. https://doi.org/10.15420/ecr.2017:7:2
44. Ghadri JR, Wittstein IS, Sharkey S et al. International Expert Consensus Document on Takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. https://doi.org/10.1093/eurheartj/ehy077
45. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. https://doi.org/10.1093/eurheartj/ehl032
46. Frangieh AH, Obeid S, Ghadri JR et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J Am Heart Assoc 2016;5:e003418. https://doi.org/10.1161/JAHA.116.003418
47. Kosuge M, Kimura K. Electrocardiographic findings of Takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J Electrocardiol 2014;47:684–9. https://doi.org/10.1016/j.jelectrocard.2014.03.004
48. Randhawa MS, Dhillon AS, Taylor HC, Sun Z, Desai MY. Diagnostic utility of cardiac biomarkers in discriminating takotsubo cardiomyopathy from acute myocardial infarction. J Card Fail 2014;20:2–8. https://doi.org/10.1016/j.cardfail.2013.12.004
49. Kurisu S, Kihara Y. Tako-tsubo cardiomyopathy: clinical presentation and underlying mechanism. J Cardiol 2012;60:429–37. https://doi.org/10.1016/j.jjcc.2012.06.015
50. Jurisic S, Camman VL, Kato K et al. Clinical predictors and prognostic impact of recovery of wall motion abnormalities in Takotsubo syndrome: results from the International Takotsubo Registry. J Am Heart Assoc 2019;8:e011194. https://doi.org/10.1161/JAHA.118.011194
51. Haghi D, Athanasiadis A, Papavassiliu T et al. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J 2006;27:2433–9. https://doi.org/10.1093/eurheartj/ehl274
52. Heggemann F, Hamm K, Brade J et al. Right ventricular function quantification in Takotsubo cardiomyopathy using two-dimensional strain echocardiography. PLoS One 2014;9:e103717. https://doi.org/10.1371/journal.pone.0103717
53. Kagiyama N, Okura H, Kume T, Hayashida A, Yoshida K. Isolated right ventricular takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:285. https://doi.org/10.1093/ehjci/jeu207
54. Liang J, Janish C, Bishu K, Reeder G. Dynamic left ventricular outflow tract obstruction in apical ballooning syndrome (Takotsubo cardiomyopathy). Perfusion 2014;30:82–4. https://doi.org/10.1177/0267659114536584
55. Bouabdallaoui N, Wang Z, Lecomte M, Ennezat PV, Blanchard D. Acute mitral regurgitation in Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2015;4:197–9. https://doi.org/10.1177/2048872614521764
56. Izumo M, Akashi YJ. Role of echocardiography for takotsubo cardiomyopathy: clinical and prognostic implications. Cardiovasc Diagn Ther 2018;8:90–100. https://doi.org/10.21037/cdt.2017.07.03
57. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in Tako-Tsubo cardiomyopathy assessed with echocardiography. QJM 2008;101:381–6. https://doi.org/10.1093/qjmed/hcn017
58. Santoro F, Stiermaier T, Tarantino N et al. Left centricular thrombi in Takotsubo syndrome: incidence, predictors, and management. Results from the GEIST (German Italian Stress Cardiomyopathy) registry. J Am Heart Assoc 2017;6:e006990. https://doi.org/10.1161/JAHA.117.006990
59. Kohan AA, Levy Yeyati E, De Stefano L et al. Usefulness of MRI in takotsubo cardiomyopathy: a review of the literature. Cardiovasc Diagn Ther 2014;4:138–46. https://doi.org/10.3978/j.issn.2223-3652.2013.10.03
60. Abbas A, Sonnex E, Pereira RS, Coulden RA. Cardiac magnetic resonance assessment of takotsubo cardiomyopathy. Clin Radiol 2016;71:e110–e119. https://doi.org/10.1016/j.crad.2015.10.020
61. Sharkey SW, Windenburg DC, Lesser JR et al. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41. https://doi.org/10.1016/j.jacc.2009.08.057
62. El-Battrawy I, Santoro F, Stiermaier T et al. Incidence and clinical impact of recurrent Takotsubo syndrome: results from the GEIST registry. J Am Heart Assoc 2019;8:e010753. https://doi.org/10.1161/JAHA.118.010753
63. Singh K, Carson K, Usmani Z, Sawhney G, Shah R, Horowitz J. Systematic review and meta-analysis of incidence and correlates of recurrence of Takotsubo cardiomyopathy. Int J Cardiol 2014;174:696–701. https://doi.org/10.1016/j.ijcard.2014.04.221
64. Campos FAD, Ritt LEF, Costa JPS et al. Factors associated with recurrence in Takotsubo syndrome: a systematic review. Arq Bras Cardiol 2020;14:477–83. https://doi.org/10.36660/abc.20180377
65. Ibanez B, James S, Agewall S et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. https://doi.org/10.1093/eurheartj/ehx393
66. Dias A, Franco E, Koshkelashvili N et al. Antiplatelet therapy in Takotsubo cardiomyopathy: does it improve cardiovascular outcomes during index event? Heart Vessels 2016;31:1285–90. https://doi.org/10.1007/s00380-015-0729-2
67. Syed FF, Asirvatham SJ, Francis J. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace 2010;13:780–8. https://doi.org/10.1093/europace/euq435
68. Brown KH, Trohman RG, Madias C. Arrhythmias in Takotsubo cardiomyopathy. Card Electrophysiol Clin 2015;7:331–40. https://doi.org/10.1016/j.ccep.2015.03.015
