Transcatheter aortic valve implantation (TAVI) is a routine procedure for patients with symptomatic severe aortic stenosis who are deemed inoperable or high-risk surgical candidates. The aim of this study was to examine real-world data on death and readmission rates in patients following the procedure.
Electronic health records for patients who underwent TAVI between April 2015 and November 2018 were reviewed. Details of the procedure, complications, length of initial hospital stay and outcomes of interest (subsequent admissions and mortality) were recorded.
In our cohort of 124 patients, the mean age was 80.8 years and 43% were male. Cardiac comorbidities were common, more than 30% had myocardial infarction (MI) and 15% had a previous coronary artery bypass graft (CABG). One in five suffered from chronic obstructive pulmonary disease (COPD), with similar prevalence of diabetes mellitus and cerebrovascular accident (CVA). In-hospital mortality was low at 3.3%, however, 30-day readmission rates were high at 14.6%; 44.4% were readmitted to hospital within one year.
TAVI is a successful procedure in Scotland with good outcome data. The potential benefit of the procedure in many patients is limited by comorbidities, which shorten life-expectancy and lead to hospital readmission. These data highlight the importance of effective multi-disciplinary discussion in a time of realistic medicine.
Introduction

Aortic stenosis (AS) is the most common primary valve disease requiring intervention in Europe and North America. The prevalence of AS increases with age, and degenerative AS is the most common type followed by AS secondary to a congenital bicuspid aortic valve.1,2 Prognosis of severe symptomatic AS is poor, with a reported 30–50% mortality at one year for patients who do not undergo any intervention.3,4
The optimal management of severe symptomatic AS in patients, often with multiple comorbidities, requires a multi-disciplinary team approach. The conservative approach with medical treatment of symptoms is associated with extremely high mortality, so valve replacement, either surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI), is the treatment of choice in suitable patients. The choice of intervention takes into account the individual’s risk of the surgery and comorbidities, and current guidelines recommend valvular intervention for patients with severe symptomatic AS, with TAVI as treatment of choice for high-risk or inoperable patients. Valvular intervention is recommended only in patients who are likely to gain improvement in their quality of life (QoL) or survival.5
Mortality benefit of TAVI has been established by randomised-controlled trials and observational studies, however, the local rates of post-procedural readmissions, mortality and length of hospital stay have not been reported. Local experience suggests that unplanned readmissions are frequent, adversely affecting the overall benefit of the procedure and are associated with significant healthcare cost. Appropriate patient selection is, therefore, essential, and the guidelines recommend heart team input, not only to optimise patient selection, but also to manage peri-procedural complications and long-term care.5
The aim of this study was to examine real-world data on post-procedural death, complications, and hospital admission rates in a cohort of Scottish patients who underwent TAVI.
Method
Study cohort
All patients who were referred for non-surgical valvular intervention were identified from the electronic health records. The study included patients residing within NHS Greater Glasgow and Clyde Health Board (NHS GGC), who underwent a TAVI procedure between 1 April 2015 and 1 November 2018 (42 months). Patients who underwent mitral valve intervention were excluded.
For each patient, electronic health records were reviewed and data were collected using a standardised data collection tool. Baseline characteristics, including echocardiography and laboratory results, along with comorbidities, were collated. Notes from the index admission were reviewed to identify peri-procedural complications and in-hospital mortality. Complications were defined as any deviation from the normal post-procedural course during index admission. Medical records for all subsequent readmissions were reviewed to determine cause and duration of inpatient stay. Readmission was defined as any new hospitalisation with a length of stay of at least one day. In addition to readmission and mortality rates, days alive and out of hospital (DAOH) were chosen as an outcome of interest. DAOH represents a patient-centred outcome, which accounts for multiple events over the course of follow-up and approximates time spent in good health. DAOH has been evaluated in patients with heart failure6 and acute coronary syndrome.7 DAOH were calculated by subtracting days in hospital and days from death until end of study follow-up from total potential follow-up time. If a patient died, the number of days from their death to the end of study follow-up (one or two years) were counted.
Statistical analysis
Baseline characteristics are presented using mean ± standard deviation (SD) for continuous variables, and frequencies or percentages for categorical variables. Data that were not normally distributed are presented as median and interquartile range. Kaplan-Meier curves were used to demonstrate outcomes. Statistical analysis was carried out using Stata 16 software package. Two-tailed level of statistical significance was set at p<0.05.
Results
Baseline characteristics
The initial screening identified 140 patients, the final dataset included 124 patients who underwent TAVI over a period of 42 months (figure 1). Baseline characteristics are presented in table 1. They were elderly (mean age 80.8 years) and there were fewer males (43.1%) than females.
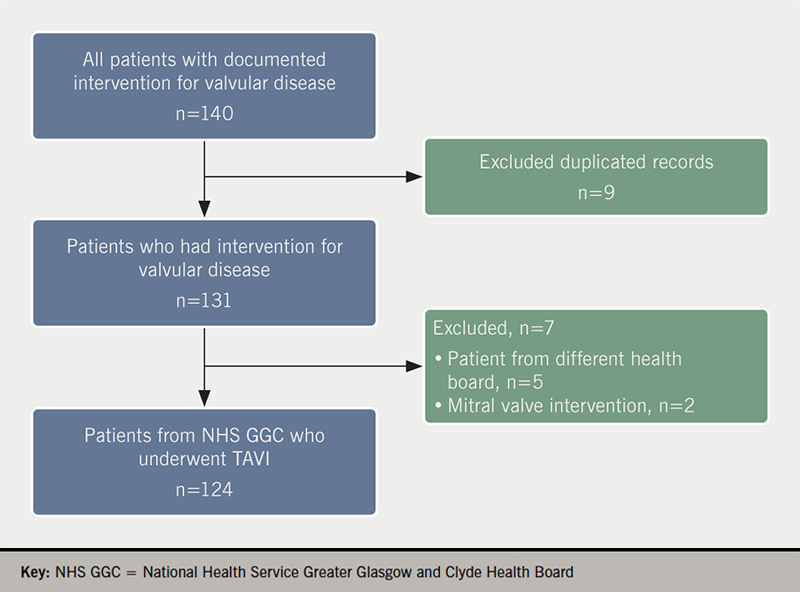
Table 1. Baseline characteristics of patients who underwent transcatheter aortic valve implantation (TAVI)
| All patients N=124 |
|
|---|---|
| Mean age, years | 80.8 ± 9.1 |
| Male, n (%) | 53 (43.1%) |
| Mean peak gradient, mmHg | 76.5 ± 24.5 |
| Mean gradient, mmHg | 44.1 ± 17.1 |
| Past medical history, n (%) | |
| Hypertension | 94 (76.4%) |
| Previous MI | 42 (34.2%) |
| Previous CABG | 19 (15.4%) |
| Previous PCI | 22 (17.9%) |
| Atrial fibrillation | 37 (30.1%) |
| Permanent pacemaker | 14 (11.4%) |
| CVA | 26 (21.1%) |
| COPD | 28 (22.8%) |
| Diabetes mellitus | 28 (22.8%) |
| eGFR <60 ml/min per 1.73m2 | 54 (47.8%) |
| LVEF ≤30% | 7 (11.7%) |
| Key: CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention | |
More than three-quarters of patients had a history of hypertension (76.4%). Over one third (34.2%) of patients had a history of myocardial infarction (MI), 19 (15.4%) had previous coronary artery bypass surgery (CABG) and 22 (17.9%) had undergone percutaneous coronary intervention (PCI). Nearly one in three patients (30.1%) had a permanent pacemaker implanted prior to TAVI. One in five patients had a history of previous cerebrovascular accident (CVA), a similar number were diagnosed with chronic obstructive pulmonary disease (COPD) and diabetes mellitus. One in seven patients (14%) was an active smoker at the time of the procedure.
Echocardiography findings
Mean ejection fraction (EF) was 53.6%, however, more than one in 10 patients had EF equal or less than 30% (data available on request). There were 25 patients with co-existing mitral regurgitation (MR); 22 (17.9%) patients had moderate and three (2.4%) had severe MR.
In keeping with the diagnosis of severe AS, mean aortic valve area (AVA) was 0.71 cm2, with peak gradient of 76.5 mmHg and mean gradient of 44.1 mmHg.
Procedure and outcomes
Procedural data are shown in table 2. Median length of stay was three days (minimum three, maximum 155 days) and nearly one in four patients (24.4%) required transfer to local hospital for a period of convalescence, ranging from one to 129 days (data available on request).
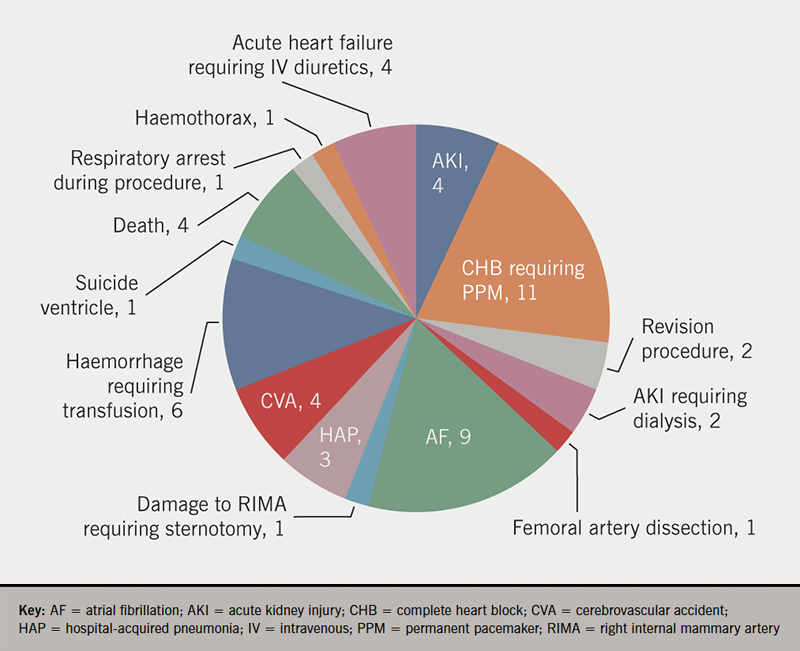
More than one third of patients experienced at least one peri-procedural complication (figure 2). Overall, four (3.3%) patients died during the index admission. The causes of death were: CVA, decompensated heart failure (HF), acute renal failure secondary to hypovolemic shock caused by post-procedural bleed, and acute lower limb and mesenteric ischaemia.
Readmission rates
Table 2. Post-procedural data and outcomes
| All patients N=124 |
|
|---|---|
| Femoral access, n (%) | 99 (80.5%) |
| Transfer to local hospital post-procedure, n (%) | 30 (24.4%) |
| Median length of hospital stay, days (Q1, Q3) | 3 (3, 6) |
| Complications, n (%) | 44 (35.8%) |
| Outcomes, n (%) | |
| In-hospital death | 4 (3.3%) |
| 30-day admission | 18 (14.6%) |
| 90-day admission | 31 (25.6%) |
| 6-month admission | 41 (34.8%) |
| 1-year admission | 52 (44.4%) |
| 2-year admission | 62 (54.9%) |
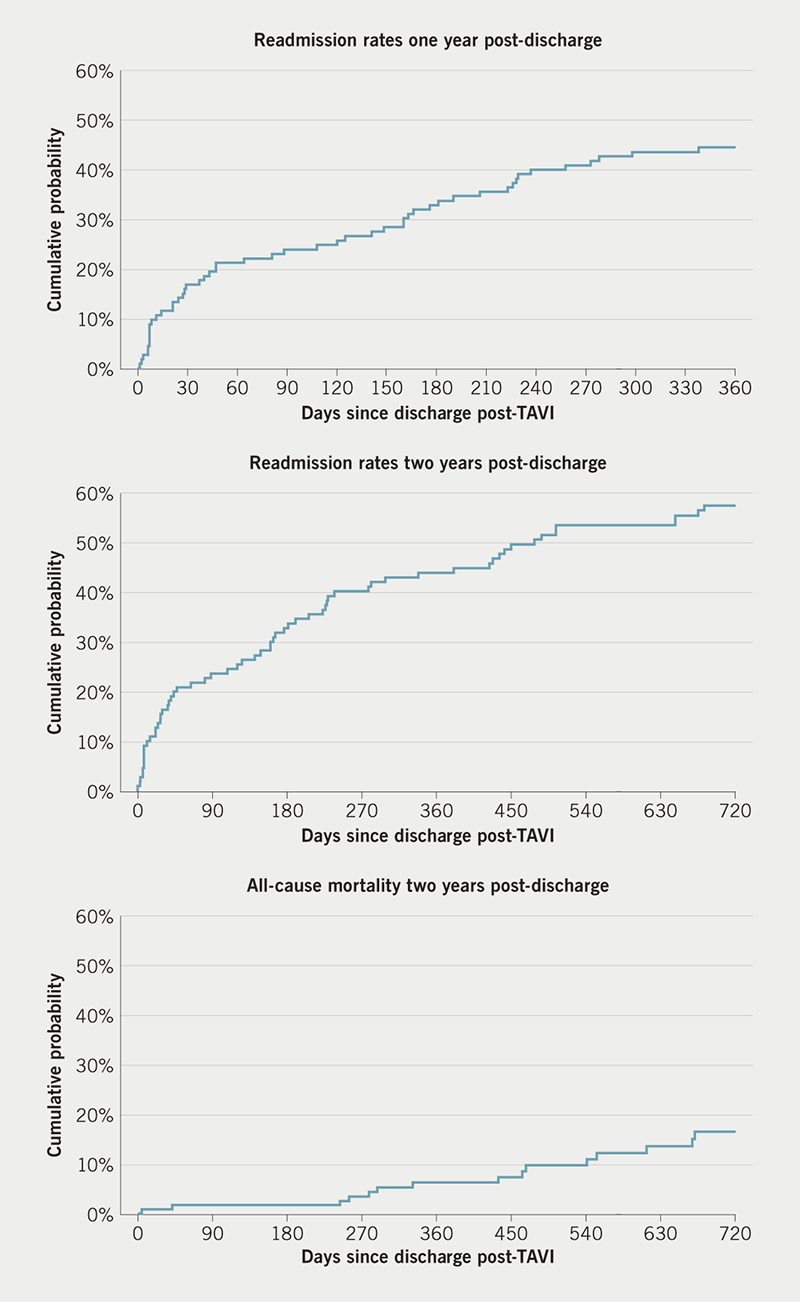
Overall, 130 readmissions occurred during the first year in 120 patients who were discharged alive post-procedure (table 2). The length of single hospital admission varied from one day to 117 days. The 30-day readmission rate was 14.6%, increasing to 25.6% at 90 days and 44.4% at one year post-procedure (table 2 and figure 3). More than half of patients (54.9%) have been readmitted to hospital within two years from the procedure (figure 3). The mean time to admission was 478 ± 118 days. There was no difference in baseline characteristics or procedural data between those who were admitted within the first or second year from the procedure (data available on request).
Nearly one in every eight patients (12.1%) had at least one admission due to cardiac cause within the first year following discharge post-TAVI, with decompensation of heart failure being the most common cause for admission (40%). Urinary and respiratory tract infection were the most common non-cardiac causes for readmissions, each caused 12% of all admissions, followed by CVA (8%) and falls (8%).
Survival and DAOH
Out of 120 patients who were discharged alive, nine patients (7.3%) died within one year and 18 (15%) died within two years from the discharge post-TAVI procedure (figure 3). The cumulative number of DAOH in the first year since discharge varied from seven to 365, with a median of 365 and mean of 336 days. For two-year follow-up, the mean number of days spent alive out of the hospital was 661.8 days, ranging from seven to 730 days.
Discussion
TAVI has become established as the treatment of choice for inoperable or high-risk patients with severe symptomatic AS and has been shown to be associated with shorter hospital stay and faster recovery, with low complication and mortality rates in these patients.2,8 The benefits of TAVI are limited by comorbidities and frailty, and previous trials comparing TAVI and SAVR have been criticised for selection bias as the numbers of screened patients were significantly higher than the number of patients enrolled in the trials and the participants did not have major comorbidities, despite advanced age. Regardless of this criticism, randomised-controlled trials, observational trials and registries have provided high-quality evidence to support the TAVI procedure.
Patient selection and risk stratification are crucial for identification of those patients who will benefit the most from the intervention. Current guidelines recommend EuroSCORE II or Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) for risk assessment. Both have been developed in a standard surgical population but neither has been validated for TAVI, nor do these scores capture significant co-existing comorbidities, which may offset the benefits of the intervention. Therefore, it is important that a multi-disciplinary heart team considers the surgical risk scoring in addition to the patient’s frailty, physiological reserve, cognition, other comorbidities and potential for improvement in QoL or life-expectancy.
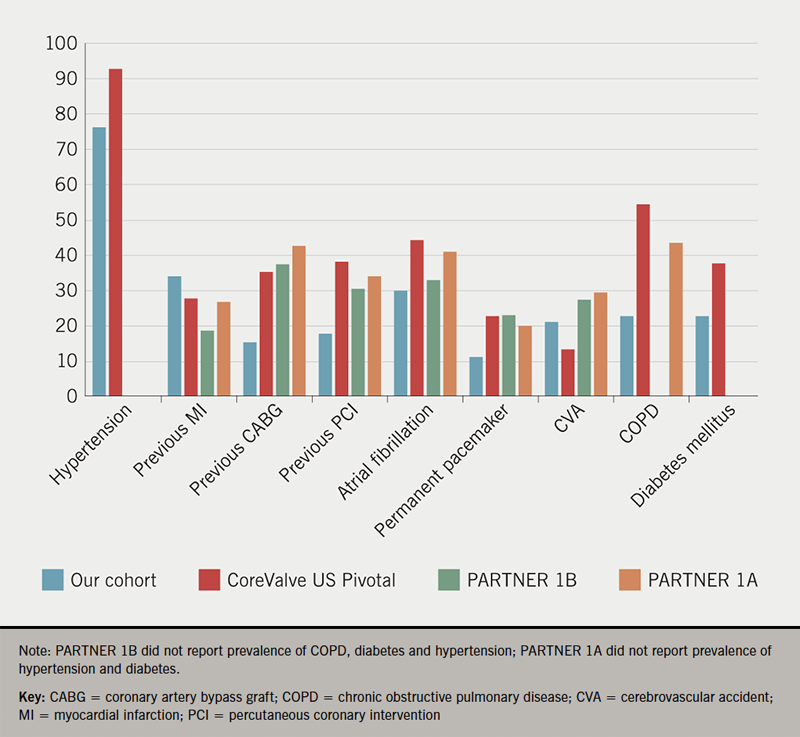
The prevalence of comorbidities was higher in our cohort than in the three pivotal trials establishing efficacy and safety of TAVI in comparison to SAVR (figure 4). COPD, left ventricular EF at discharge and estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73m2 have been identified as independent predictors of early post-discharge mortality. The National Readmissions Database identified >4 comorbidities and chronic lung disease as independent predictors of early readmissions,8 and nearly a quarter of our group (24.2%) had four comorbidities or more. Frailty and disability have also been found to have a major impact on 30-day and one-year mortality rates after TAVI.9
In our cohort, nine patients (7.3%) died within one year from the procedure, which is lower than mortality rates among TAVI patients reported in the PARTNER (Placement of Aortic Transcatheter Valves) 1A,10 PARTNER 1B3 and CoreValve US Pivotal9 trials (24.2%, 30.7% and 22.8%, respectively). The in-hospital mortality of 3.3% was in keeping with other real-world registries.11,12 Similar to other reports, all in-hospital deaths were procedure related.13
Despite these good procedural outcomes, the readmission rates were high among our cohort of patients. Out of those who were alive at discharge, 14.6% of patients were readmitted within 30 days, 44.4% within the first year and 54.9% within two years post-TAVI. There were 12.1% of patients having at least one admission due to cardiac cause in the first year of the follow-up. This is almost three times more than short-term, and double the long-term readmission rates observed in the PARTNER study (5.6% at 30 days and 22.3% at one year in PARTNER 1B, 4.4% at 30 days and 18.2% at one year in PARTNER 1A).3,10
This real-life data show higher rates of readmission than those reported in the randomised-controlled clinical trials. The short-term readmission rate of 14.6% is in keeping with those observed in the National Readmission Database and other trials.8,14-16 Our long-term readmission rate was either similar or higher than other European reports; the Bern TAVI registry observed only 25.4% of patients readmitted to hospital within one year post-TAVI, with 46.1% of admissions due to cardiovascular cause,17 whereas Nombela-Franco and colleagues observed a similar number of admissions to our cohort (43.9% one-year readmission rate), with a significant proportion of admissions due to cardiac causes (41.1%).16
A longer post-procedural length of stay was associated with increased one-year readmission rates,18 and we have noted a similar trend in our cohort, however, due to a low number of subjects, this was not formally tested.
Major complications (CVA, haemorrhage, need for sternotomy or para-valvular leak requiring revision procedure) were infrequent (8.1%) and the rate of permanent pacemaker implantation was 11%, in keeping with the lower end of the reported figures. Risk of cerebrovascular events remains one of the main concerns associated with TAVI. In our study, four patients (3.2%) suffered from CVA, which is consistent with reported rates of 3–4%.19
A proportion of our patients spent a significant length of time in the hospital during subsequent readmissions, leading to a diminished benefit of TAVI, but also significant cost. The ADVANCE study evaluated the cost-effectiveness of TAVI in high-risk patients in comparison with standard care, based on PARTNER 1B results. The incremental cost-effectiveness ratio (ICER) per quality adjusted life-year (QALY) was calculated at £18,577, below the UK National Institute for Health and Care Excellence (NICE) cost-effectiveness threshold of £20,000.20 These calculations were based on an assumption of a one-year hospitalisation rate of 14.6% post-TAVI and a fixed hospitalisation rate of 0.668 events per month. Our one-year readmission rate was nearly three times that in PARTNER 1B, limiting the cost-effectiveness of the intervention and overall benefit to the patient. Most importantly, the high admission rate was driven by non-cardiac admissions, emphasising the need for a holistic approach to the patient and recognition of other competing, non-cardiac, causes of morbidity among these high-risk patients.
Limitations
Our cohort included patients from one Scottish health board and the relatively small sample size prohibits detailed subgroup analysis and multi-variate analysis to identify predictors for readmission and to assess association between readmission and mortality. We relied on electronic medical notes and, infrequently, the data were limited or missing. Additionally, STS-PROM and EuroScore II were not documented and individual components of the risk assessment were not routinely available in the electronic records. Due to different IT systems, admissions to non-Scottish health boards were not captured, however, from the records reviewed there was no suggestion that any of the patients have been admitted to a non-Scottish hospital during the two-year follow-up.
Conclusion
Our outcomes for TAVI in high risk and inoperable patients are good with low peri-procedural mortality and low major complication rates. However, the short- and long-term readmission rates were high, reducing the potential benefit of the intervention and emphasising the need for holistic assessment of these patients during the selection process.
Key messages
- Transcatheter aortic valve implantation (TAVI) has become established as a frequently performed procedure for high-risk patients with symptomatic aortic stenosis with good procedural outcomes
- Real-life patients undergoing TAVI have more comorbidities than trial patients and a high readmission rate in the subsequent two years
- Careful selection of patients is of paramount importance to maximise the benefits of TAVI
Conflicts of interest
None declared.
Funding
None.
Study approval
This project was approved by the local research review department.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. https://doi.org/10.1016/S0140-6736(06)69208-8
2. Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8:162–72. https://doi.org/10.1038/nrcardio.2010.202
3. Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. https://doi.org/10.1056/NEJMoa1008232
4. Carabello BA. Evaluation and management of patients with aortic stenosis. Circulation 2002;105:1746–50. https://doi.org/10.1161/01.CIR.0000015343.76143.13
5. Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Kardiol Pol 2018;76:1–62. https://doi.org/10.5603/KP.2018.0013
6. Ariti CA, Cleland JGF, Pocock SJ et al. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J 2011;162:900–06. https://doi.org/10.1016/j.ahj.2011.08.003
7. Fanaroff AC, Cyr D, Neely ML et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes 2018;11:e004755. https://doi.org/10.1161/CIRCOUTCOMES.118.004755
8. Kolte D, Khera S, Sardar MR et al. Thirty-day readmissions after transcatheter aortic valve replacement in the United States: insights from the Nationwide Readmissions Database. Circ Cardiovasc Interv 2017;10:e004472. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004472
9. Hermiller JB, Yakubov SJ, Reardon MJ et al. Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:343–52. https://doi.org/10.1016/j.jacc.2016.04.057
10. Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. https://doi.org/10.1056/NEJMoa1103510
11. Walther T, Hamm CW, Schuler G et al. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol 2015;65:2173–80. https://doi.org/10.1016/j.jacc.2015.03.034
12. Mack MJ, Brennan JM, Brindis R et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA 2013;310:2069–77. https://doi.org/10.1001/jama.2013.282043
13. Saia F, Latib A, Ciuca C et al. Causes and timing of death during long-term follow-up after transcatheter aortic valve replacement. Am Heart J 2014;168:798–806. https://doi.org/10.1016/j.ahj.2014.07.023
14. Hannan EL, Samadashvili Z, Jordan D et al. Thirty-day readmissions after transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis in New York State. Circ Cardiovasc Interv 2015;8:e002744. https://doi.org/10.1161/CIRCINTERVENTIONS.115.002744
15. Panaich SS, Arora S, Patel N et al. Etiologies and predictors of 30-day readmission and in-hospital mortality during primary and readmission after transcatheter aortic valve implantation. Am J Cardiol 2016;118:1705–11. https://doi.org/10.1016/j.amjcard.2016.08.052
16. Nombela-Franco L, Trigo M Del, Morrison-Polo G et al. Incidence, causes, and predictors of early (≤30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2015;8:1748–57. https://doi.org/10.1016/j.jcin.2015.07.022
17. Franzone A, Pilgrim T, Arnold N et al. Rates and predictors of hospital readmission after transcatheter aortic valve implantation. Eur Heart J 2017;38:2211–17. https://doi.org/10.1093/eurheartj/ehx182
18. Sud M, Qui F, Austin PC et al. Short length of stay after elective transfemoral transcatheter aortic valve replacement is not associated with increased early or late readmission risk. J Am Heart Assoc 2017;6:e005460. https://doi.org/10.1161/JAHA.116.005460
19. Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129–38. https://doi.org/10.4244/EIJV8I1A20
20. Brecker S, Mealing S, Padhiar A et al. Cost-utility of transcatheter aortic valve implantation for inoperable patients with severe aortic stenosis treated by medical management: a UK cost-utility analysis based on patient-level data from the ADVANCE study. Open Heart 2014;1:e000155. https://doi.org/10.1136/openhrt-2014-000155
