Loperamide is an over-the-counter, peripherally-acting, µ-opioid receptor agonist commonly used in the treatment of diarrhoea. It has increasingly been recognised as a potential drug of misuse, having previously been thought to have low potential for abuse owing to its low bioavailability and poor penetration of the central nervous system. High doses can result in life-threatening cardiac-toxicity.
We present a case of a young woman who had been self-treating her depression with high doses of loperamide for one year, who then presented to hospital with syncope secondary to ventricular tachycardia (VT). While in the emergency department (ED) the patient had monomorphic pulseless VT requiring electrical cardioversion multiple times. Transfer to a tertiary cardiac centre was immediately arranged after she was stabilised and intubated. This complicated the diagnostic process as a thorough history could not be obtained on arrival to the tertiary centre, which meant the loperamide misuse only came to light multiple days into admission, after the patient was extubated. The final diagnosis of loperamide-induced secondary long-QT syndrome was made and the patient made a full recovery.
Introduction
Loperamide-induced cardiac toxicity is rare but potentially fatal. We describe a case of long-term overdose in a vulnerable young adult, leading to a near-fatal cardiac event.
Case
A 34-year-old woman was referred to a tertiary centre cardiology team with multiple episodes of pulseless ventricular tachycardia (VT) requiring direct current (DC) cardioversion. The patient initially presented to a district general hospital (DGH) with a seizure preceded by palpitations. In the emergency department (ED) she had three witnessed episodes of pulseless VT, each requiring a single 200 J shock before achieving return of spontaneous circulation. The residual rhythm after the final shock was sinus bradycardia with marked electrical conduction disturbances.
The patient’s only past medical history of significance was depression and anxiety, for which she had not received prescribed pharmacological treatment in the preceding nine months. The initial review elicited no risk factors for ventricular arrhythmia. She was a non-smoker; drank minimal alcohol; had no illicit drug use and no family history of cardiac channelopathies or sudden cardiac death syndrome. The results of investigations are shown in table 1.
Table 1. Investigations
| Investigation | Result |
|---|---|
| Echocardiography | Structurally and functionally normal heart |
| Cardiac catheterisation | Normal unobstructed coronary arteries with no evidence of coronary artery dissection |
| Cardiac magnetic resonance | Unremarkable with normal ejection fraction |
| Family pedigree | No abnormality |
| Biochemistry panel including thyroid stimulating hormone (TSH) and extended electrolyte screen | Normal |
Review of the admission electrocardiogram (ECG) (figure 1) demonstrated a sinus rhythm, right-axis deviation, left posterior hemi-fascicular block and a prolonged QTc interval. The QRS complex appearances in the right and mid-precordial leads demonstrated right-bundle-branch-block morphology and a relatively broad r-prime. This led to conflicting differential diagnoses of Brugada’s syndrome or long-QT (LQT) syndrome. A “Brugada” ECG at one inter-costal space higher was not consistent with a Brugada pattern. However, the QT-interval remained consistently and significantly prolonged. Despite the episodes of monomorphic VT, and although non-sustained polymorphic VT was seen (figure 2), the diagnosis was changed to primary LQT syndrome. The patient was initially commenced on isoprenaline at the DGH for treatment of presumed Brugada syndrome and electrical storm. This was switched to intravenous esmolol for treatment of presumed LQT syndrome on arrival at the tertiary care centre. However, the final diagnosis of loperamide-induced secondary LQT syndrome was made following a more detailed history from the patient herself (after she was extubated on day four of admission), uncovering the misuse of loperamide. The patient was switched to oral nadolol 80 mg daily with a plan to gradually down-titrate and stop in three to six months. Initial considerations of a life-vest were abandoned following normalisation of the QTc-interval during the admission.
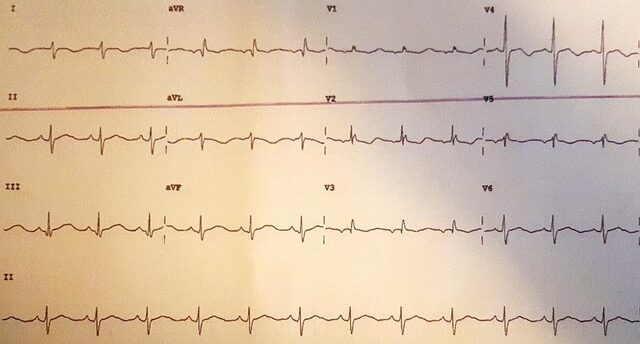
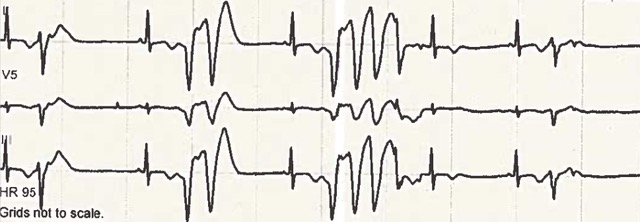
As it is acknowledged that medication-induced electrical-conduction abnormalities can uncover or exacerbate pre-existing genetic channelopathies. A detailed family pedigree was obtained, which revealed no abnormality.
Discussion
After first documentation in 1970, the misuse of loperamide has become increasingly common.1 This is alarming given the high reported mortality rates when taken above recommended doses (3–21%).2,3
Numerous reports describe cardiac conduction abnormalities thought to be secondary to loperamide, including bradycardia, prolonged QTc, ventricular arrythmias and cardiac arrest.4-7 The exact mechanism of cardiac toxicity is not fully understood. It is thought that the inhibition of the sodium/potassium transmembrane ion channels in the cardiac cells has an important role. Kang et al., in 2016, simulated loperamide testing on the two most abundant repolarising potassium channels (KvLQT1/mink and the hERG channel) and the most common cardiac sodium channel (Nav1.5). This experiment demonstrated that loperamide inhibited all three channels, but with different affinities, with an extremely high inhibitory action on the hERG channel.8 Similar results were also demonstrated in a study by Klein et al.9 The effects on the sodium and potassium channels are thought to be the cause of the changes noted on ECG, notably QRS broadening and elongation of QTc.
Loperamide has an average half-life of 10.8 hours.10 However, it is hypothesised that in supra-therapeutic doses it may saturate the metabolic pathway, significantly extending the half-life; with one study demonstrating a half-life of 34.8 hours.11
From the patient’s perspective, she was completely unaware of any side effects of loperamide and felt it was significantly improving her mood when compared with previously taking selective serotonin reuptake inhibitors (SSRIs), hence, the continued and prolonged use of the over-the-counter medication and self-discontinuing the SSRI she was previously prescribed.
The Medicine and Health Regulatory Agency (MHRA) have issued safety alerts over the use of loperamide, acknowledging that at high doses it may cause cardiac conduction abnormalities that could potentially be fatal.12 In the UK, loperamide is currently available over-the-counter with a maximum recommended dose of 16 mg/day. Following the MHRA safety alert, the British Intestinal Failure Alliance (BIFA) issued a statement providing guidance. They advocate the use of loperamide in patients with high output stomas in doses up to 80 mg/day, but recommend ECG monitoring in doses exceeding 16 mg/day.13 Therefore, it is reasonable to hypothesise that loperamide has a good cardiac-safety margin when used at recommended daily doses, but appears to have a substantial arrhythmic burden when misused. Further research is required to ascertain at what doses loperamide begins to affect the cardiac action potential.
Conclusion
This is a case of acquired LQT secondary to loperamide toxicity leading to a near fatal cardiac event, which also highlights the importance of a thorough medical history. Loperamide misuse is increasing and in supra‑therapeutic doses can lead to potentially fatal ventricular arrythmias. Our patient made a full recovery and, prior to discharge, she was educated on the subject, including written advice on which QTc-prolonging medication to avoid. She was also reviewed by the psychiatry team who arranged for community follow-up. Given the potentially fatal side effect profile and high mortality rates in overdose, it should be considered to label loperamide as a prescription-only medication.
Conflicts of interest
None declared.
Funding
None.
Patient consent
The patient provided written consent for publication. All attempts were made to ensure patient confidentiality was maintained.
References
1. Daniulaityte R, Carlson R, Falck R et al. “I just wanted to tell you that loperamide WILL WORK”: a web-based study of extra-medical use of loperamide. Drug Alcohol Depend 2013;130;241–4. https://doi.org/10.1016/j.drugalcdep.2012.11.003
2. Lasoff DR, Koh CH, Corbett B et al. Loperamide trends in abuse and misuse over 13 years: 2002–2015. Pharmacotherapy 2017;37:249–53. https://doi.org/10.1002/phar.1885
3. Food and Drugs Administration. FDA warns about serious heart problems with high doses of the antidiarrheal medicine loperamide (Imodium), including from abuse and misuse. Safety Announcement. Available at: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-warns-about-serious-heart-problems-high-doses-antidiarrheal-medicine [accessed 6 July 2016].
4. Parker BM, Rao T, Matta A et al. Loperamide induced cardiac arrhythmia successfully supported with veno-arterial ECMO (VA-ECMO), molecular adsorbent recirculating system (MARS) and continuous renal replacement therapy (CRRT). Clin Toxicol 2019;57:1118–22. https://doi.org/10.1080/15563650.2019.1580370
5. Eggleston W, Nacca N, Marraffa JM. Loperamide toxicokinetics: serum concentrations in the overdose setting. Clin Toxicol 2015;53:495–6. https://doi.org/10.3109/15563650.2015.1026971
6. Rasla S, St. Amand A, Garas MK et al. Unexpected serious cardiac arrhythmias in the setting of loperamide abuse. R I Med J (2013) 2017;100:33–6. Available from: http://rimed.org/rimedicaljournal/2017/04/2017-04-33-case-rasla.pdf
7. Akel T, Bekheit S. Loperamide cardiotoxicity: a brief review. Ann Noninvasive Electrocardiol 2017;23:1–4. https://doi.org/10.1111/anec.12505
8. Kang J, Compton DR, Vaz RJ, Rampe D. Proarrhythmic mechanisms of the common anti-diarrheal medication loperamide: revelations from the opioid abuse epidemic. Naunyn Schmiedebergs Arch Pharmacol 2016;389:1133–7. https://doi.org/10.1007/s00210-016-1286-7
9. Klein MG, Haigney MC, Mehler PS, Fatima N, Flagg TP, Krantz MJ. Potent inhibition of hERG channels by the over-the-counter antidiarrheal agent loperamide. JACC Clin Electrophysiol 2016;2:784–9. https://doi.org/10.1016/j.jacep.2016.07.008
10. Killinger JM, Weintraub HS, Fuller BL. Human pharmacokinetics and comparative bioavailability of loperamide hydrochloride. J Clin Pharmacol 1979;19:211–18. https://doi.org/10.1002/j.1552-4604.1979.tb01654.x
11. Eggleston W, Nacca N, Marraffa JM. Loperamide toxicokinetics: serum concentrations in the overdose setting. Clin Toxicol 2015;53:495–6. https://doi.org/10.3109/15563650.2015.1026971
12. Medicines and Healthcare Products Regulatory Agency. Loperamide (Imodium): reports of serious cardiac adverse reactions with high doses of loperamide associated with abuse or misuse. Drug Safety Update 2017;11:2. Available from: https://www.gov.uk/drug-safety-update/loperamide-imodium-reports-of-serious-cardiac-adverse-reactions-with-high-doses-of-loperamide-associated-with-abuse-or-misuse
13. Nightingale J, Meade U, BIFA committee. The use of high dose loperamide in patients with intestinal failure. British Intestinal Failure Alliance (BIFA) position statement. Redditch: BAPEN, April 2018. Available from: https://www.bapen.org.uk/pdfs/bifa/position-statements/use-of-loperamide-in-patients-with-intestinal-failure.pdf
