Mitral regurgitation is a common valvular heart disorder increasing with age. Many patients are ineligible for mitral valve surgery due to their age and other comorbidities. Left untreated, patients develop severe disease with a poor prognosis. The development of lower risk percutaneous mitral valve interventions has helped meet the needs of this previously untreated patient group. This review explores the recent and more established developments that have expanded the armamentarium for transcatheter mitral valve intervention.
Introduction
In western populations, mitral regurgitation (MR) is the second most common valvular heart disorder, after aortic stenosis, with a reported prevalence of approximately 2%. This prevalence increases with age and affects up to 10% of those over the age of 75.1,2 Severe MR tends to run an insidious yet malignant course and, without intervention, confers a poor prognosis. Untreated, it is associated with progressive left ventricular remodelling, and ultimately dysfunction, resulting in increased morbidity and an annual mortality rate of 5%.3-5
Mitral valve surgery (MVS), typically mitral valve repair (MVr), for severe degenerative MR is associated with favourable outcomes and is considered the gold-standard therapeutic option.6,7 Despite this, almost one half of patients referred for surgery are deemed ineligible due to advanced age, comorbidities and ventricular dysfunction.8 In contrast, surgical intervention for functional MR remains a topic of conjecture. MVS in this population confers a high operative mortality, a significant risk of recurrent MR and has not been proven to improve survival.9,10 For these reasons, the majority of patients with severe MR remain undertreated and are not offered curative intervention. This increasingly evident unmet need has fuelled the development of minimally invasive and lower risk transcatheter-based therapies to treat MR.
Percutaneous mitral valve intervention now presents a valuable therapeutic option for patients at high or prohibitive surgical risk with degenerative or functional MR.
Mitral valve anatomy and classification
The mitral valve is a complex anatomical structure comprising two mitral leaflets, the annular attachment at the atrioventricular junction, the chordae tendineae, and the papillary muscles arising from the left ventricular myocardium. The valve apparatus is intricate and relies heavily on the functional integrity of its components to prevent regurgitant blood flow from the left ventricle into the left atrium during systole. Pathology at any level of the apparatus can, therefore, render the valve incompetent.11,12
MR can be classified into degenerative or functional forms. Degenerative (or primary) mitral regurgitation (DMR) refers to a structural abnormality of the valve leaflets or chordae tendineae. Degenerative mitral valve disease, causing prolapse or a flail leaflet, is the most common aetiology of MR in the developed world. It is largely attributable to fibro-elastic deficiency or myxomatous infiltration resulting in chordal stretching or rupture. In contrast to DMR, functional (or secondary) MR (FMR) occurs in the absence of organic mitral valve disease, but instead as a result of left ventricular distortion due to ischaemic or non-ischaemic cardiomyopathy.6,7,13-15 The well-established Carpentier classification system describes the motility of the mitral valve leaflets relative to the mitral annular plane and enables distinction between underlying aetiologies of MR (figure 1).16
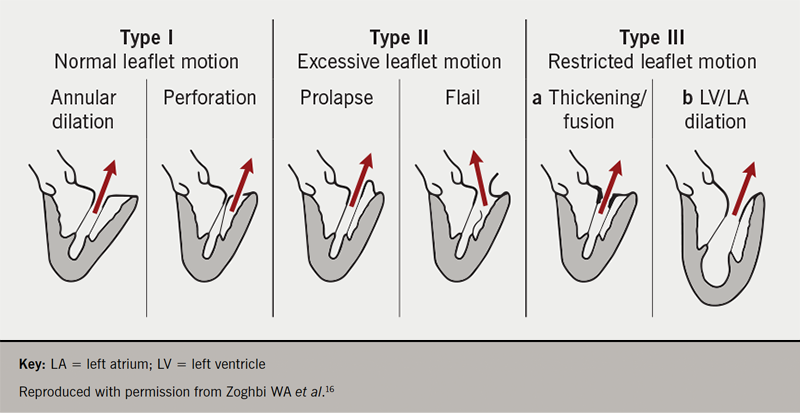
Current guidelines and unmet need
In patients with severe DMR, there is no evidence that even aggressive medical therapy will alter the natural history of disease and improve prognosis. The indication, therefore, for surgical intervention is clear in DMR, and it is associated with excellent outcomes in experienced centres.6,7,17 Timing of intervention is critical as the sequelae of MR, including left ventricular systolic dysfunction (LVSD) and pulmonary hypertension, may herald the onset of symptoms but, additionally, indicate higher risk. The onset of symptoms alone can, therefore, render the older or comorbid patient unsuitable for MVS, as is evidenced by almost 50% of patients with severe DMR being denied surgery in the UK.8,18,19
While MVS in DMR is established, the invasive management of FMR remains less certain. As the valve incompetence in FMR is ultimately due to altered left ventricular geometry, it is less obvious whether interventions targeting the mitral valve are beneficial. MVS in this group, in the absence of concomitant surgical revascularisation, carries a high operative risk and has failed to demonstrate improved survival. Whether FMR itself is a driver of disease progression or whether it simply reflects the severity of LVSD remains unclear, however, selected patients do benefit from mitral valve intervention suggesting a role for correction.20-23
Historically, the aforementioned limitations have culminated in a large proportion of patients being left untreated.8 This has led to the recognition of a striking ‘unmet need’ and has drawn focus on the development of transcatheter-based techniques that might be less invasive and, therefore, safer for this high-risk population. Over the last two decades, evolving percutaneous MV interventions have demonstrated increasingly satisfying results and now serve as a lifeline for prohibitive or high surgical risk patients with DMR/FMR. Despite this, International guidelines have lagged behind the technology and only now are transcatheter options being recommended to bridge the gap and address this unmet need. Most recently, the 2020 American College of Cardiology (ACC)/American Heart Association (AHA) guideline for the management of patients with valvular heart disease has upgraded to 2a its recommendation for transcatheter edge-to-edge repair (TEER) in severely symptomatic patients (New York Heart Association [NYHA] class III/IV) with severe DMR and high or prohibitive surgical risk, where MV anatomy is favourable and patient life-expectancy is at least one year. The same body also now recommends TEER in symptomatic, medically optimised patients with FMR, with European guidance likely to follow suit in the coming year.6,7 More recently, following the impressive results from COAPT (Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients Trial) discussed below, the 2021 European Society of Cardiology (ESC) guidelines for the diagnosis and management of acute and chronic heart failure have consolidated the role for TEER in FMR. They too have provided a class 2a recommendation for TEER in patients with FMR who remain symptomatic despite guideline directed medical therapy.23
Transcatheter mitral valve intervention
The current percutaneous methods available mimic the concepts of already well-established surgical techniques and include TEER, ring annuloplasty, new chordal implantation, as well as complete mitral valve replacement. A plethora of novel devices are currently under exploration; however, this review will be limited to those devices with current CE mark approval and those that are commercially available in the UK (figure 2, table 1).24-26
Figure 2. Transcatheter techniques including transcatheter edge-to-edge repair, chordal implantation, annuloplasty and replacement. Devices here have obtained CE mark approval
| Transcatheter mitral valve intervention | |
|---|---|
| Edge-to-edge repair | |
MitraClip system
|
PASCAL repair system
|
| Chordal implantation | |
NeoChord DS1000
|
HARPOON system
|
| Annuloplasty | |
| Direct Cardioband mitral system 
|
Indirect Carillon system 
|
| Transcatheter mitral valve replacement | |
Tendyne system
|
|
Table 1. Utility of transcatheter technique according to aetiology of mitral regurgitation (MR), as described by Carpentier’s classification16
| Carpentier classification | ||||
|---|---|---|---|---|
| Type I | Type II | Type IIIa | Type IIIb | |
| TEER | + | +++ | – | ++ |
| Chordal implantation | – | +++ | – | – |
| Annuloplasty | +++ | – | – | + |
| TMVR | ++ | ++ | + | +++ |
| Key: TEER = transcatheter edge-to-edge repair; TMVR = transcatheter mitral valve replacement | ||||
Multi-disciplinary team and patient selection
The management of mitral valve disease can be complex, and, therefore, warrants a dedicated multi-disciplinary care team (MDT) in an experienced centre to ensure optimal management. The mitral valve heart team usually consists of at least one cardiac surgeon, an interventional cardiologist with experience in catheter-based mitral valve therapies, an imaging cardiologist with expertise in advanced echocardiography and a cardiac anaesthetist, working alongside the centre’s heart failure team. Together they offer a tailored approach to management, taking into consideration the aetiology and risk profile of the patient.27
Medically optimised patients with severe MR, regardless of aetiology, who have a prohibitive or high surgical risk can be considered for transcatheter mitral valve intervention. Pre-procedural echocardiographic assessment of MV anatomy and pathology is of utmost importance to ascertain feasibility of transcatheter repair and guide suitability. Additional specialist investigations such as gated computed tomography (CT) are also now becoming routine.
Transcatheter edge-to-edge repair
The transcatheter edge-to-edge repair (TEER) technique is the most frequently used and researched method for percutaneous mitral valve repair. The edge-to-edge repair approximates the anterior and posterior leaflets, usually creating a double-orifice mitral valve. This technique replicates the open surgical approach that was first performed in 1991 by Ottavio Alfieri, ensuring a fixed area of systolic co-aptation while maximising preservation of the annular function and subvalvular apparatus. TEER is the only guideline-recommended percutaneous therapy available for the treatment of degenerative or functional MR.28,29
MitraClip system
The MitraClip system (Abbott Vascular, Santa Clara, CA, USA) is currently the only US Food and Drug Administration (FDA) approved device for transcatheter mitral valve repair and received CE approval in 2008. The device has now been used to treat over 150,000 patients worldwide.30 MitraClip is implanted in small numbers in approximately 20 centres in the UK and is currently commissioned, for DMR cases only, in three dedicated centres.
The MitraClip is a two-armed cobalt-chromium device with a polyester covering that promotes tissue growth between leaflets. The system is transeptally delivered into the left atrium under fluoroscopic and transoesophageal echocardiography (TOE) guidance. Once in the left atrial chamber, the device is navigated towards the valve at the point of regurgitation and the clip arms are opened perpendicular to the line of MV co-aptation. The system is advanced into the left ventricle and then retracted in order to grasp and securely capture the corresponding mitral leaflets. Once the arms are closed, the quality of co-aptation and reduction of regurgitation can be evaluated, and the device can be withdrawn or moved before irretrievable clip deployment (figure 3). Clips can be repositioned to obtain optimal reduction, and additional clips may be placed if residual MR persists and if the resultant valve area and trans-mitral gradient will allow.
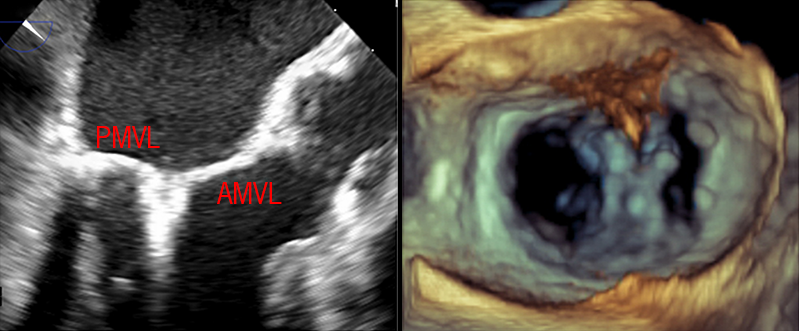
As experience with the MitraClip system has increased, the ‘Everest’ anatomical criteria have become less relevant as procedural success has been demonstrated in more complex anatomy. The cohort of patients that may benefit from the MitraClip device has increased as generational improvements have resulted in an enhanced delivery system (NTR) and longer arms (XTR) to improve reach and facilitate leaflet grasping. Most recently, the advent of the fourth generation MitraClip device has brought with it a wider clip to tackle broad flails (NTW/XTW) and independent gripper function to enable sequential leaflet grasping.
Evidence base
TEER for the management of severe MR has been the subject of extensive observational study and favourable outcomes have been demonstrated with the MitraClip device. When scrutinised against MVS in the EVEREST II trial (The Endovascular Edge-to-Edge Repair Study), although surgery was more effective at reducing the severity of MR initially, rates of reduction in MR at 12 and 24 months were similar. The MitraClip system demonstrated comparable improvements in left ventricular dimensions and NYHA functional class to MVS, suggesting clinical efficacy. MitraClip also demonstrated a favourable safety profile, with a major adverse event rate of 15% compared with 48% in the MVS group.31-35 Despite these encouraging results, the EVEREST II study is hampered by its lack of real-world relevance. The trial involved a predominance of patients with DMR, who by trial design, were also eligible for surgery. In real-world practice, however, the DMR patients treated with MitraClip worldwide are generally not surgical candidates and, therefore, represent a much higher risk group than that studied in the EVEREST II trial. Since then, several large European registries have scrutinised MitraClip therapy in the real world and have mitigated this concern by successfully demonstrating its efficacy and safety in this high-risk cohort. The Transcatheter Valve Treatment Sentinel Pilot Registry of 628 patients confirmed overall acute procedural success (defined as a reduction in MR severity to ≤2 without complications) in 95.4% of patients and was similar in DMR and FMR groups. Along with a reduction in severity of MR, the registry confirmed an improvement in NYHA functional class to ≤2 in most patients (74.9% of FMR, and 76.5% of DMR).34 This high rate of technical success and improvement in NYHA functional class is consistent with other large registries, including ACCESS-EU (A two phase Observational Study of the MitraClip System in Europe)35 and TRAMI (Transcatheter Mitral Valve Interventions) Registry.36
COAPT versus MITRA-FR
Two randomised-controlled trials have studied the use of MitraClip in patients with heart failure and severe FMR. MITRA-FR (Multi-centre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients with Severe Secondary Mitral Regurgitation) and COAPT randomised patients to guideline-directed medical therapy (GDMT) or GDMT plus MitraClip. The trials presented discrepant results, with MITRA-FR concluding a neutral primary outcome, and COAPT demonstrating clear superiority of MitraClip over GDMT alone.37,38 Although initially disconcerting, a number of trial differences including strictness and monitoring of GDMT, study duration and intra-procedural results have been suggested to explain the discrepant results. Another common theory has highlighted important differences in patient selection, including baseline characteristics, which may account for the contradictory results. Patients enrolled in the MITRA-FR trial had more severe LV dilatation (LV end-diastolic volume [LVEDV] 252 ml) with proportionately less MR (effective regurgitant orifice area [EROA] 0.31 cm2). Conversely, the COAPT investigators enrolled patients with smaller LV volumes (LVEDV 192 ml), possibly more likely to reverse remodel, but with more severe MR (EROA 0.41 cm2).
During a swell of discussion following the publication of these two studies, Grayburn et al. have proposed that those with MR deemed disproportionate to the degree of LV dilatation, more akin to the COAPT population, may preferentially benefit from restoring mitral valve function.39,40 Recent studies, however, now dispute this theory and suggest the discrepant outcomes may not be so easily explained. As careful patient selection appears to be imperative, COAPT-like patients have been defined according to the inclusion/exclusion criteria used in the trial and should guide the Mitral Valve Heart Team as to what patients are likely to benefit. The criteria include symptomatic MR grade >3+, LVEF>20%, LV end-systolic dimension <70 mm and MV orifice area ≥4 cm2.
The extended RESHAPE-HF2 study (A Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation) is currently recruiting and randomising participants to either GDMT, or GDMT plus MitraClip. With a targeted population closer to COAPT than MITRA-FR, it is hoped that this study will provide further evidence and clarity regarding the optimal management of patients with significant FMR and chronic heart failure.41
PASCAL system
The PASCAL system (Edwards Lifesciences, Irvine, CA, USA) purports to overcome some of the perceived limitations of the MitraClip device and to increase the number of patients who might benefit from TEER. The broader paddles aim to facilitate leaflet capture and the presence of a central spacer functions to both fill the regurgitant orifice and yet reduce the risk of iatrogenic mitral stenosis. Additionally, the device has an independent grasping mechanism, whereby one leaflet can be captured before focusing on the other. These unique features of the PASCAL device may overcome some of the anatomical constraints that render some patients less suitable for the MitraClip system, for example short posterior leaflets and prolapse gaps greater than 10 mm. In the UK, the awaited fourth generation MitraClip system has been modified to compete with some of the positive features of PASCAL.42
Results from the CLASP study, which was designed to assess the safety and efficacy of the PASCAL system, demonstrated promising clinical and echocardiographic outcomes at short- and long-term follow-up. Of the 62 patients enrolled, severity grade of MR was reduced to ≤1 in 82%, with evidence of reverse LV remodelling. Interestingly, two-thirds of the patients enrolled had FMR, consolidating the concept that treating mitral valve incompetence in selected patients with FMR can improve clinical outcomes.42-44 PASCAL is commercially available in the UK and is commissioned by NHS England for treatment of DMR at its specified sites.
Transapical chordal implantation
Transapical ‘off-pump’ chordal implantation is an alternative for selected patients with leaflet prolapse. Access to the left ventricle is obtained via a left lateral mini-thoracotomy under TOE guidance. The device is directed towards the MV plane and synthetic, expanded polytetrafluoroethylene chords are advanced through the prolapsing leaflet segment. A knot on the atrial surface ensures fixation of the chord to the leaflet. This process can be repeated until the desired number of chords have been implanted. The neo-chords are then progressively tensioned, until the natural area of co-aptation between the anterior and posterior mitral leaflets is restored. Once adequate reduction in MR has been achieved, the chordae are secured to the epicardial surface of the ventricular access site. Two systems have currently obtained the CE mark.
NeoChord DS 1000
The NeoChord device (NeoChord, Inc., St. Louis Park, MN, USA) was initially evaluated in The TransApical Chordae Tendineae (TACT) trial, which confirmed feasibility of this new technique. More recently, early outcomes of the device were evaluated in the NeoChord Independent International Registry. Results were encouraging with a low risk of procedural complication and a significant reduction in MR. Outcomes were maintained at one year, with the majority having mild or less MR, clinically significant LV reverse remodelling and an improvement in NYHA functional class. These results were achieved on a beating heart without the need for cardiopulmonary bypass, however, the applicability of the device may be limited by selective anatomical criteria.45-48
Harpoon TDS-5
An initial feasibility trial of the Harpoon Mitral Valve Repair System (Edwards Lifesciences, Irvine, CA, USA) demonstrated safe implantation of neo-chordae resulting in a reduction in MR severity to moderate or less in 90% of patients. At six-month follow-up, 92% of patients had sustained improvement in MR (moderate or less), and a NYHA functional class of I. The introducing catheter of the Harpoon device is smaller in diameter compared with NeoChord and contains a haemostatic valve. The system is, therefore, considered to reduce the risk of intra-operative bleeding and the need for blood transfusion, an end point encountered by 16% of patients in the TACT trial.49
Annuloplasty
Direct annuloplasty – Cardioband
Regardless of aetiology, annular dilatation and remodelling is frequently a contributing factor to the degree of MR. Percutaneous catheter-based annuloplasty, therefore, has a promising role to fill the therapeutic gap for high surgical risk patients, either as a stand-alone procedure or as an adjunct to other mitral valve intervention. The Cardioband (Edwards Lifesciences, Irvine, CA, USA) is an adjustable Dacron band that is directly implanted on to the atrial surface of the posterior mitral annulus and then cinched to reduce annular dimensions and improve co-aptation. This transeptal, yet time-consuming, technique aims to mimic the surgical annuloplasty ring and replicate its positive outcomes in selected patients. A multi-centre trial following patients with moderate-to-severe FMR treated with the Cardioband system, demonstrated a reduction in anteroposterior diameter of >30% and an improvement in MR grade to ≤2 in 86.3% at six months.50-52 There have been concerns regarding a risk of coronary artery injury during deployment. The observed rate of injury is approximately 3%, secondary to direct interaction between the anchors of the device and the circumflex artery.52 Further device modifications and more thorough pre-procedural CT imaging have claimed to reduce this risk.
Indirect annuloplasty – Carillon
Indirect annuloplasty takes advantage of the anatomical proximity of the coronary sinus to the mitral annulus. An intravascular device is delivered to the coronary sinus and by applying external tension, shrinks and remodels the mitral annulus to improve co-aptation. The Carillon mitral contour system (Cardiac Dimensions, Kirkland, WA, USA) is currently the only indirect device to have achieved CE mark approval. The TITAN trial revealed that the Carillon system has a good safety profile with significant reductions in MR and improvements in functional status. Despite these propitious results, applicability can be challenging as anatomy is often distorted in FMR increasing the distance between the coronary sinus and mitral annulus. Furthermore, risk of compression of the left circumflex artery, which also lies in direct proximity, is an important limitation and may preclude indirect annuloplasty in some patients.53,54 The sham-controlled REDUCE-FMR trial demonstrated a significant reduction in MR and improvement in LV volumes with Carillon compared with the sham-operated group, with an 84% successful device implant rate.55 A larger double-blinded controlled trial is ongoing.
Transcatheter mitral valve replacement – Tendyne
The success and rapid adoption of transcatheter aortic valve replacement, segued the evolution of transcatheter mitral valve replacement (TMVR) to further expand the treatment options available for severe MR. Development, however, has been slower and more challenging owing to the heterogeneity of MR and the anatomical complexity. Although many devices are in varying stages of development, the Tendyne (Abbott Vascular, Santa Clara, CA, USA) mitral valve implantation is the first of its kind to have gained commercial clearance and CE mark approval. Tendyne is a transcatheter porcine bioprosthetic mitral valve replacement that is delivered trans-apically under TOE and fluoroscopic guidance. The tri-leaflet prosthesis is comprised of a circular inner frame and an outer frame shaped to resemble the mitral annulus. It is secured using a tether attached to an epicardial pad and tension is applied to ensure optimal seating of the prosthesis (figure 4).
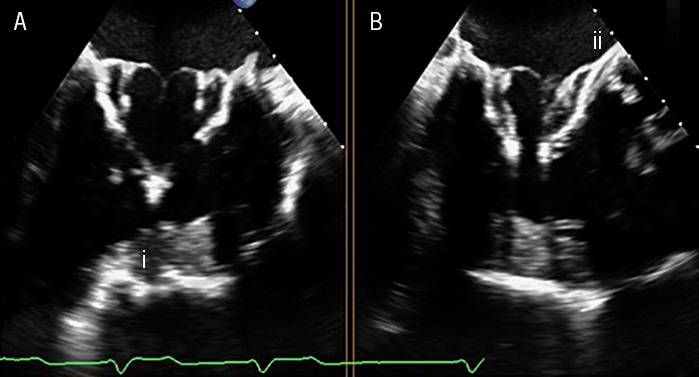
In a global feasibility trial, Tendyne implantation was safe and eliminated MR in 98% of patients at one-year follow-up. Despite elimination of MR, the study observed a one-year mortality of 26% suggesting that further larger studies are required to assess the long-term clinical success of TMVR.56,57 The pivotal SUMMIT trial is currently ongoing to compare the safety and efficacy of the Tendyne mitral valve replacement to MitraClip in prohibitive surgical risk patients with DMR/FMR.58
Conclusion
Transcatheter mitral valve intervention is a rapidly evolving field and has generated extensive interest in the past two decades. Despite this, many patients in the UK with severe MR are still undertreated. Multiple percutaneous devices are now available to treat patients that are not eligible for surgery, with increasingly promising results to support their role. A dedicated Heart Team is crucial, regardless of aetiology, to assess risk and advise a tailored management approach.
Further investigation is required to consolidate the clinical efficacy of novel therapies and identify those patients most likely to benefit. Transcatheter mitral intervention is likely to hold the key to addressing an unmet clinical need and will be an invaluable addition to the interventional cardiologist’s toolbox.
Key messages
- The prevalence of mitral regurgitation (MR) is growing, however, it remains undertreated in a large cohort of patients. Without intervention severe MR confers a poor prognosis
- For severe degenerative MR, surgical mitral valve repair remains the gold-standard therapeutic option and can restore a normal life-expectancy
- For severe symptomatic patients that have a high or prohibitive surgical risk, recent guidelines have upgraded their recommendation for percutaneous edge-to-edge repair to 2a
Conflicts of interest
RG: none declared. RS: proctoring/consultation fees and travel grants received from Abbott (US).
Funding
The BJC commissioned RG and RS, who received an honorarium for writing this article. The article was sponsored by Abbott Laboratories (see sponsorship statement).
References
1. Iung B, Baron G, Butchart E et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–43. https://doi.org/10.1016/S0195-668X(03)00201-X
2. Nkomo VT, Gardin JM, Skelton TN et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. https://doi.org/10.1016/S0140-6736(06)69208-8
3. Trochu J, Dillon R, Gustafsson F et al. Mitral regurgitation – unmet need for improved management strategies. Int J Cardiol Heart Vasc 2014;5:26–41. https://doi.org/10.1016/j.ijcha.2014.10.011
4. Prakash R, Horsfall M, Markwick A et al. Prognostic impact of moderate or severe mitral regurgitation (MR) irrespective of concomitant comorbidities: a retrospective matched cohort study. BMJ Open 2014;4:e004984. https://doi.org/10.1136/bmjopen-2014-004984
5. Enriquez-Sarano M, Avierinos J, Messika-Zeitoun D et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. ACC Curr J Rev 2005;14:17. https://doi.org/10.1016/j.accreview.2005.05.039
6. Vahanian A, Alfieri O, Andreotti F et al. Guidelines on the management of valvular heart disease (version 2012). Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33:2451–96. https://doi.org/10.1093/eurheartj/ehs109
7. Otto CM, Nishimura RA, Bonow RO et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021;143:e72–e227 (published online 17th December 2020). https://doi.org/10.1161/CIR.0000000000000923
8. Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–65. https://doi.org/10.1093/eurheartj/ehm001
9. Calafiore A, Iacò A, Gallina S et al. Surgical treatment of functional mitral regurgitation. Int J Cardiol 2013;166:559–71. https://doi.org/10.1016/j.ijcard.2012.05.027
10. Asgar A, Mack M, Stone G. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231–48. https://doi.org/10.1016/j.jacc.2015.02.009
11. McCarthy K, Ring L, Rana B. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr 2010;11:i3–i9. https://doi.org/10.1093/ejechocard/jeq153
12. Perloff J, Roberts W. The mitral apparatus. Circulation 1972;46:227–39. https://doi.org/10.1161/01.CIR.46.2.227
13. Vahanian A, Baumgartner H, Bax J et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230–68. https://doi.org/10.1093/eurheartj/ehm095
14. Carpentier A. Cardiac valve surgery – the “French correction”. J Thorac Cardiovasc Surg 1983;86:323–37. https://doi.org/10.1016/S0022-5223(19)39144-5
15. Monteagudo Ruiz J, Galderisi M, Buonauro A et al. Overview of mitral regurgitation in Europe: results from the European Registry of mitral regurgitation (EuMiClip). Eur Heart J Cardiovasc Imaging 2018;19:503–07. https://doi.org/10.1093/ehjci/jey011
16. Zoghbi WA, Adams D, Bonow RO et al. Recommendations for noninvasive evaluation of native valvular regurgitation. A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echo 2017;30:303-70. http://doi.org/10.1016/j.echo.2017.01.007
17. Lazam S, Vanoverschelde J, Tribouilloy C, Grigioni F, Suri R, Avierinos J. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation. Circulation 2017;135:410–22. https://doi.org/10.1161/CIRCULATIONAHA.116.023340
18. Rosenhek R, Rader F, Klaar U et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006;113:2238–44. https://doi.org/10.1161/CIRCULATIONAHA.105.599175
19. Suri RM, Vanoverschelde JL, Grigioni F et al. Association between early surgical intervention versus watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609–16. https://doi.org/10.1001/jama.2013.8643
20. Rossi A, Dini FL, Faggiano P et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675–80. https://doi.org/10.1136/hrt.2011.225789
21. Grigioni F, Enriquez-Sarano M, Zehr K, Bailey K, Tajik A. Ischemic mitral regurgitation. Long-term outcome and prognostic implications with quantitative Doppler assessment. ACC Curr J Rev 2001;10:33. https://doi.org/10.1016/S1062-1458(01)00387-7
22. Goliasch G, Bartko P, Pavo N et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2017;39:39–46. https://doi.org/10.1093/eurheartj/ehx402
23. McDonagh T, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
24. Denti P, Sala A, Belluschi I, Alfieri O. Over 15 years: the advancement of transcatheter mitral valve repair. Ann Cardiothorac Surg 2021;10:15–27. https://doi.org/10.21037/acs-2020-mv-18
25. Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol 2014;63:2057–68. https://doi.org/10.1016/j.jacc.2014.01.039
26. Del Forno B, De Bonis M, Agricola E et al. Mitral valve regurgitation: a disease with a wide spectrum of therapeutic options. Nature Rev Cardiol 2020;17:807–27. https://doi.org/10.1038/s41569-020-0395-7
27. Külling M, Corti R, Noll G et al. Heart team approach in treatment of mitral regurgitation: patient selection and outcome. Open Heart 2020;7:1–10. https://doi.org/10.1136/openhrt-2020-001280
28. Maisano F, Torracca L, Oppizzi M et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg 1998;13:240–6. https://doi.org/10.1016/S1010-7940(98)00014-1
29. Lorusso R, Borghetti V, Totaro P, Parrinello G, Coletti G, Minzioni G. The double-orifice technique for mitral valve reconstruction: predictors of postoperative outcome. Eur J Cardiothorac Surg 2001;20:583–9. https://doi.org/10.1016/S1010-7940(01)00828-4
30. Abbott Cardiovascular. MitraClip transcatheter mitral valve repair. Available at: https://www.cardiovascular.abbott/us/en/hcp/products/structural-heart/transcatheter-valve-solutions/mitraclip.html
31. Feldman T, Kar S, Rinaldi M et al.; EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686–94. https://doi.org/10.1016/j.jacc.2009.03.077
32. Feldman T, Foster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–406. https://doi.org/10.1056/NEJMoa1009355
33. Glower D, Kar S, Trento A et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172–81. https://doi.org/10.1016/j.jacc.2013.12.062
34. Nickenig G, Estevez-Loureiro R, Franzen O et al. Percutaneous mitral valve edge-to-edge repair: in hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875–84 https://doi.org/10.1016/j.jacc.2014.06.1166
35. Maisano F, Franzen O, Baldus S et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052–61. https://doi.org/10.1016/j.jacc.2013.02.094
36. Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J 2016;37:703–12. https://doi.org/10.1093/eurheartj/ehv627
37. Obadia JF, Messika-Zeitoun D, Leurent G et al.; MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. https://doi.org/10.1056/NEJMoa1805374
38. Stone GW, Lindenfeld J, Abraham WT et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–18. https://doi.org/10.1056/NEJMoa1806640
39. Grayburn P, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation. JACC Cardiovasc Imaging 2019;12:353–62. https://doi.org/10.1016/j.jcmg.2018.11.006
40. Packer M, Grayburn P. Contrasting effects of pharmacological, procedural, and surgical interventions on proportionate and disproportionate functional mitral regurgitation in chronic heart failure. Circulation 2019;140:779–89. https://doi.org/10.1161/CIRCULATIONAHA.119.039612
41. Clinicaltrials.gov. A randomised study of the MitraClip device in heart failure patients with clinically significant mitral regurgitation (RESHAPE-HF). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01772108
42. Corpataux N, Winkel M, Kassar M et al. The PASCAL device – early experience with a leaflet approximation device: what are the benefits/limitations compared with the MitraClip? Curr Cardiol Rep 2020;22:74. https://doi.org/10.1007/s11886-020-01305-1
43. Praz F, Spargias K, Chrissoheris M et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet 2017;390:773–80. https://doi.org/10.1016/S0140-6736(17)31600-8
44. Lim DS, Kar S, Spargias K et al. Transcatheter valve repair for patients with mitral regurgitation: 30-day results of the CLASP study. JACC Cardiovasc Interv 2019;12:1369–78. https://doi.org/10.1016/j.jcin.2019.04.034
45. Seeburger J, Rinaldi M, Nielsen S et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation. J Am Coll Cardiol 2014;63:914–19. https://doi.org/10.1016/j.jacc.2013.07.090
46. Colli A, Manzan E, Aidietis A et al. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation†. Eur J Cardiothorac Surg 2018;54:460–66. https://doi.org/10.1093/ejcts/ezy064
47. Colli A, Manzan E, Besola L et al. One-year outcomes after transapical echocardiography-guided mitral valve repair. Circulation 2018;138:843–5. https://doi.org/10.1161/CIRCULATIONAHA.118.033509
48. Kiefer P, Meier S, Noack T et al. Good 5-year durability of transapical beating heart off-pump mitral valve repair with neochordae. Ann Thorac Surg 2018;106:440–5. https://doi.org/10.1016/j.athoracsur.2018.01.092
49. Gammie J, Bartus K, Gackowski A et al. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device. J Am Coll Cardiol 2018;71:25–36. https://doi.org/10.1016/j.jacc.2017.10.062
50. Nickenig G, Schueler R, Dager A et al. Treatment of chronic functional mitral valve regurgitation with a percutaneous annuloplasty system. J Am Coll Cardiol 2016;67:2927–36. https://doi.org/10.1016/j.jacc.2016.03.591
51. Nickenig G, Hammerstingl C, Schueler R et al. Transcatheter mitral annuloplasty in chronic functional mitral regurgitation: 6-month results with the cardioband percutaneous mitral repair system. JACC Cardiovasc Interv 2016;9:2039–47. https://doi.org/10.1016/j.jcin.2016.07.005
52. Messika-Zeitoun D, Nickenig G, Latib A et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur Heart J 2018;40:466–72. https://doi.org/10.1093/eurheartj/ehy424
53. Bail D. Treatment of functional mitral regurgitation by percutaneous annuloplasty using the Carillon Mitral Contour System – currently available data state. J Interv Cardiol 2017;30:156–62. https://doi.org/10.1111/joic.12370
54. Siminiak T, Wu JC, Haude M et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN trial. Eur J Heart Fail 2012;14:931–8. https://doi.org/10.1093/eurjhf/hfs076
55. Witte KK, Lipiecki J, Siminiak T et al. The REDUCE FMR trial: a randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail 2019;7:945–55. https://doi.org/10.1016/j.jchf.2019.06.011
56. Del Val D, Ferreira-Neto A, Wintzer-Wehekind J et al. Early experience with transcatheter mitral valve replacement: a systematic review. J Am Heart Assoc 2019;8:e013332. https://doi.org/10.1161/JAHA.119.013332
57. Regueiro A, Granada JF, Dagenais F et al. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol 2017;69:2175–92. https://doi.org/10.1016/j.jacc.2017.02.045
58. ClinicalTrials.gov. Clinical trial to evaluate the safety and effectiveness of using the Tendyne mitral valve system for the treatment of symptomatic mitral regurgitation. Available at: https://clinicaltrials.gov/ct2/show/NCT03433274
