Atrial fibrillation (AF) is a major cause of recurrent stroke and transient ischaemic attack (TIA) in the UK. As many patients can have asymptomatic paroxysmal AF, prolonged arrhythmia monitoring is advised in selected patients following a stroke or TIA. This service evaluation assessed the clinical and potential health economic impact of prolonged arrhythmia monitoring post-stroke using R-TEST monitoring devices.
This was a prospective, case-controlled, service evaluation in a single health board in the North of Scotland. Patients were included if they had a recent stroke or TIA, were in sinus rhythm, and did not have another indication for, or contraindication to, oral anticoagulation. A health economic model was developed to estimate the clinical and economic value delivered by the R-TEST monitoring. Approval to use anonymised patient data in this service evaluation was obtained.
During the evaluation period, 100 consecutive patients were included. The average age was 70 ± 11 years, 46% were female. Stroke was the presenting complaint in 83% of patients with the other 17% having had a TIA. AF was detected in seven of 83 (8.4%) patients who had had a stroke and one of 17 (5.9%) patients with a TIA. Health economic modelling predicted that adoption of R-TEST monitoring has a high probability of demonstrating both clinical and economic benefits.
In conclusion, developing a post-stroke arrhythmia monitoring service using R-TEST devices is feasible, effective at detecting AF, and represents a probable clinical and economic benefit
Introduction

Cerebrovascular disease is a major cause of disability and mortality in adults worldwide.1 Patients can present with a stroke or transient ischaemic attack (TIA). Due to the risk of recurrent events, early investigation and treatment of risk factors is advised.2,3 One of the major risk factors for stroke is atrial fibrillation (AF). AF is a common cardiac arrhythmia, which is estimated to affect 2.5% of the adult population in Scotland, with a large proportion undiagnosed and consequently untreated. Cardioembolism accounts for around a quarter of all ischaemic strokes, which is most commonly caused by AF.4 Current evidence shows that, in up to 24% of all patients presenting with AF-induced ischaemic stroke or TIA, the event is the first clinical documentation of AF.5,6 The clinical demand is significant because AF-related strokes are also associated with greater stroke severity and a poorer prognosis.7,8 Without adequate treatment of AF with oral anticoagulants, the annual risk of stroke recurrence in these patients also remains high (~20%).9,10 However, the risk of stroke in AF patients following appropriate anticoagulation with warfarin is reduced by 45–69%,9,11 and additional benefits may be achieved with direct oral anticoagulants.12
While many patients will have permanent or persistent AF, others will have intermittent paroxysmal AF (pAF; AF interspersed with periods of normal sinus rhythm), which can make diagnosis difficult, especially if the periods of AF are asymptomatic. In patients with pAF the risk of stroke is similar to permanent AF,13 and most guidelines do not differentiate between AF and pAF with regard to anticoagulation. Nevertheless, this is an area of research interest, on account of the increasing awareness that the total burden of AF correlates with stroke risk.14
It is estimated that asymptomatic pAF is present in up to 16% of patients who have had a stroke, although rates vary depending on the mechanism and duration of monitoring.15,16 This has led to a change in national guidelines, which now advise post-stroke arrhythmia monitoring for at least 24 hours in cases where cause of stroke is not obvious and where anticoagulation may be considered,2 although longer periods may be beneficial to increase detection rates.6 Due to the risk of early recurrent stroke and the evidence supporting timely anticoagulation,3 monitoring should be undertaken without undue delay. However, the duration of monitoring and implementation of these guidelines remains inconsistent and is debated for a variety of historical and logistical reasons.17
In our centre, an arrhythmia monitoring service has been established for stroke and TIA patients in sinus rhythm at the time of presentation, not known to have AF or pAF, or any other indication for anticoagulation, and with no absolute contraindications to anticoagulation. Patients are monitored for ~7 days with an R-TEST device to identify patients with AF/pAF and to allow subsequent intervention with anticoagulation.
The aim of this project was to evaluate this post-stroke arrhythmia monitoring service in terms of clinical effectiveness (identification of patients with AF and appropriate anticoagulation) and potential health economic benefit.
Methods
Design and setting
This was a prospective, case-control service evaluation in patients who had a newly diagnosed stroke or TIA. Evaluation took place in Raigmore Hospital, the principal hospital of the NHS Highland Health Board, serving a predominantly remote and rural population in the North of Scotland. The health board is geographically the largest in Scotland covering 32,500 km2 and providing care for ~320,000 people.
Participants
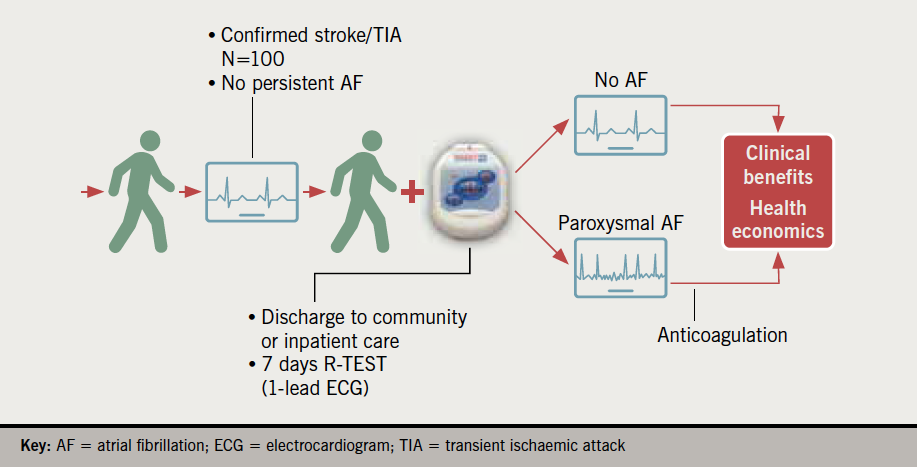
Inpatients and outpatients diagnosed with non-haemorrhagic stroke or TIA between 20 June 2019 and 3 July 2020 were considered for this study. Patients were included if they were in sinus rhythm at the time of presentation, not known to have AF (or pAF) or another indication for anticoagulation, and with no absolute contraindications to anticoagulation (figure 1). All eligible referrals from the stroke unit were accepted for monitoring.
Protocol
Patients were identified by a member of the stroke team and referred for a R-TEST monitor (figure 2) (R-TEST 4, Novacor UK Ltd.) either as an inpatient or outpatient. Seven devices were purchased for this study and subsequent clinical use. The monitors were fitted by a member of the cardiac physiology team using a standard technique. The R-TEST was configured to a dedicated AF/pAF algorithm (which relies on RR analysis combining wavelet transform and fractal analysis) with electrocardiogram (ECG) strips being recorded from 60 s pre- until 40 s post-AF detection (total ECG strip length of 100 s). A maximum of 14 strips could be recorded for AF events (during the recording period). The device was able to report the presence of AF, AF burden (%), longest episode of AF and the time from fitting the monitor to detection of AF. Other auto-detection parameters to aid in the detection of AF were ‘relative pauses’, which were triggered when an R–R interval was detected that had a duration 175% of the preceding R–R interval, and supraventricular ectopy (SVE) – including isolated beats, couplets, triplets, and runs (paroxysmal supraventricular tachycardia [PSVT]), which were triggered when an R–R interval was detected that was at least 25% shorter than the preceding R–R interval. Auto-detection parameters for ‘absolute pauses’ (R–R interval of >3 s), ventricular tachycardia (VT), ventricular ectopy (VE) (including isolated beats, couplets, and triplets), tachycardias (>120 bpm), and bradycardias (<50 bpm) were also programmed. Aside from auto-detection parameters, the patient was able to make up to five patient-activated recordings (20 s pre- and 10 s post-button press) if they were symptomatic. The maximum length of monitoring was set as seven days, but was shortened if AF was detected sooner or patients were discharged early from hospital. When returned, the devices were analysed by a cardiac physiologist and a report sent to the requesting clinician. The R-TEST monitors were decontaminated following manufacturer’s guidelines and local trust policy.
Data collection and handling
Anonymised data were entered into Microsoft Excel 2013 (Microsoft, Redmond, WA, USA) and analysed using SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, USA). Chi-squared statistics and Student’s t-test were used as appropriate for non-parametric and parametric data, respectively. A p value <0.05 was considered statistically significant.
Health economic modelling
A health economic model was developed to estimate the potential clinical and economic value delivered by R-TEST monitoring. Clinical and economic proxies were used to estimate the annual net benefit in terms of patient outcome and pounds Sterling (£). These proxies included the number of secondary strokes, daily cost of inpatient care, number of inpatient bed days, device purchase and replacement every three years, pharmaceutical anticoagulation costs, and staff costs associated with R-TEST device fitting, monitoring, upkeep, and cleaning.
Ethical considerations
Approval to use anonymised patient data in this service evaluation was obtained from the local Caldicott Guardian. Full ethical approval was not required for this post hoc service evaluation.
Results
Demographics
One hundred patients were referred for an R-TEST monitor during the study period, of which 46% were female. The average age was 70 ± 11 years. Stroke was the presenting complaint in 83% of patients, with the other 17% presenting with TIA. Inpatients made up 54% of the referrals (table 1A).
Table 1A. Outcomes: stroke versus transient ischaemic attack (TIA)
| All (N=100) |
Stroke (N=83) |
TIA (N=17) |
p value | |
|---|---|---|---|---|
| Mean age ± SD, years [range] | 70 ± 11 [48–89] |
71 ± 11 [48–89] |
65 ± 12 [48–89] |
0.053 |
| Female, n (%) | 46 (46%) | 38 (46%) | 8 (47%) | 0.923 |
| AF detected, n (%) | 8 (8%) | 7 (8.4%) | 1 (5.9%) | 0.724 |
| Inpatient, n (%) | 54 (54%) | 52 (63%) | 2 (12%) | <0.001 |
| Key: AF = atrial fibrillation; SD = standard deviation | ||||
During the same time period (20 June 2019 and 3 July 2020), the clinical service admitted 341 patients to the hospital with a non-haemorrhagic stroke. Of these, 87 had pre-existing AF prior to admission, with a further 60 cases of AF diagnosed on admission. In addition, 268 patients presented with a TIA. Of these, 31 had pre-existing AF and a further 16 AF diagnoses were made at the clinic. Thus, there were 194 non-haemorrhagic stroke and 221 TIA patients who did not have known AF (415 patients in total).
AF detection
In the 100 stroke or TIA patients who did not have known AF and were referred for an R-TEST, AF was detected in seven (8.4%) of 83 patients who had had a stroke and one (5.9%) of 17 patients with a TIA. Similarly, five (9.3%) of 54 inpatients and three (6.5%) of 46 outpatients were identified with pAF following R-TEST monitoring. There was no statistical difference between those with AF detected or not in terms of age, gender or diagnosis (all p>0.05) (table 1B).
Table 1B. Differences between patients with and without atrial fibrillation (AF) detected
| AF negative (N=92) |
AF positive (N=8) |
p value | |
|---|---|---|---|
| Mean age ± SD, years [range] | 69 ± 12 [48–89] |
74 ± 8 [64–82] |
0.310 |
| Female, n (%) | 44 (48%) | 2 (25%) | 0.214 |
| Stroke, n (%) | 76 (82.6%) | 7 (87.5%) | 0.724 |
| Inpatient, n (%) | 49 (53.3%) | 5 (62.5%) | 0.645 |
| Key: SD = standard deviation | |||
The AF burden in the eight patients with AF detected ranged from 2 to 100%. The time of onset ranged from 0 to over 212 hours (table 2).
Table 2. Characteristics of patients diagnosed with new AF (or paroxysmal AF)
| Patient | Age, years | Gender, M/F | Stroke/TIA | IP/OP | AF burden, % | Time from fitting of monitor to detection, days:hours | Longest episode, hours:mins:secs |
|---|---|---|---|---|---|---|---|
| 1 | 65 | M | Stroke | IP | 2 | 05:13 | 00:30:13 |
| 2 | 82 | M | Stroke | IP | 56 | 00:00 | 05:54:00 |
| 3 | 81 | M | Stroke | IP | 48 | 04:17 | 00:05:55 |
| 4 | 73 | F | Stroke | OP | 42 | 00:00 | 00:01:05 |
| 5 | 67 | F | Stroke | IP | 100 | 00:00 | 212:40:00 |
| 6 | 64 | M | TIA | OP | 4 | 01:21 | 01:45:00 |
| 7 | 85 | M | Stroke | OP | 92 | 00:00 | – |
| 8 | 76 | M | Stroke | IP | 16 | – | – |
| Key: AF = atrial fibrillation; F = female; IP = inpatient; M = male; OP = outpatient; TIA = transient ischaemic attack | |||||||
Health economic analysis
The incidence of AF in patients enrolled in this study (5.9–8.4%) was used to derive a health economic model based on the annual number of NHS Highland patients presenting with stroke or TIA and not known to be in AF (415 in total), an estimated secondary stroke risk of 15 to 30% in patients with undiagnosed and untreated AF,9,10 and a 66% reduction in secondary stroke risk in AF patients following anticoagulation.18,19 Based on these figures, between four and 11 patients with undiagnosed and untreated AF are predicted to have a secondary stroke within one year of their initial stroke or TIA. Implementation of R-TEST monitoring and anticoagulation is predicted to prevent three to seven of these secondary strokes (figure 3). The intervention is, therefore, projected to deliver a reduction of 78–182 inpatient bed days and annual gross savings of £31,200 to £72,800. Accounting for additional R-TEST purchase and replacement costs (n=8 devices at £2,000 each), ECG data analysis and clinical time (at £20 per patient), as well as anticoagulant drug costs (£683.75 per patient), the achievable net savings is estimated at a maximum of £35,235.75 each year, or £5,033 per secondary stroke prevented (table 3).
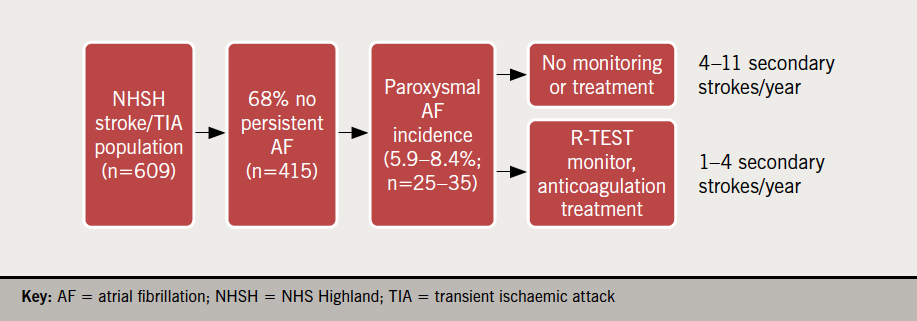
Table 3. Health economic analysis
| Secondary care costs | |||
|---|---|---|---|
| Category | Per stroke patient | ||
| No intervention (n=4–11 stroke patients) |
With R-TEST intervention (n=1–4 stroke patients) | ||
| Bed days | 26 | 104–286 | 26–104 |
| Annual inpatient treatment cost per day | £400 | £41,600–£114,400 | £10,400–£41,600 |
| Intervention costs | |||
| Category | Cost per patient | Total annual cost | |
| R-TEST device purchase and replacement (n=8) | £12.85 (n=415) | £5,333 | |
| R-TEST data analysis and clinical time | £20 (n=415) | £8,300 | |
| Anticoagulant drug cost | £683.75 (n=25–35) | £17,093.75–£23,931.25 | |
| Total annual intervention cost | £30,726.75–£37,564.25 | ||
Discussion
This service evaluation has demonstrated that establishing a post-stroke prolonged monitoring service (up to seven days) is feasible within existing staff resources in an NHS environment. Overall, we detected AF (or pAF) in 8% of monitored patients. In addition to the improved patient care the service provides, our health economic analysis suggests a net financial benefit to the organisation.
Identifying asymptomatic AF and pAF has been identified in national guidelines as an important part of clinical care to reduce future strokes and associated morbidity and mortality.2 Nevertheless, robust guidance and data for physicians are lacking. In our study, we identified AF or pAF in 8.4% of eligible patients with stroke and 5.9% of eligible patients with TIA. This is comparable with other data,5 however, it is lower than a recent audit from Kishore and colleagues20 who detected new AF in 14.7% of eligible post-ischaemic stroke patients. Detection rates may be even greater where even longer periods of monitoring are employed or where implantable devices such as Medtronic LINQ are used.17 While Kishore et al. detected a greater, but non-significant, number of new AF cases for inpatients versus outpatients (19.4% vs. 5.7%, p=0.07), our detection rates were similar irrespective of inpatient/outpatient status (9.3% vs. 6.5%). It is likely that the increased age (median age 76 years) and number of comorbidities in their inpatient population contributed to the greater overall detection rates of new AF than that of the present study.
Interestingly, in the present study there were no differences in age and gender between patients with and without AF, which confirms that predicting patients at risk of AF is difficult.
The number of patients recruited to this pilot study was less than might be predicted to require a R-TEST in clinical practice. This is likely due to a variety of factors that were out of the scope of this study to measure. These include patients already on a direct oral anticoagulant (DOAC) for other reasons, e.g. venous thrombosis or pulmonary embolism, patients deemed too frail for a DOAC, patients deemed unable to comply with monitoring, and variation in practice, including under-referral by clinicians. Under-referral by clinicians might be assumed to improve once the full clinical service is established. In the MonDAFIS (MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke) study, strokes were classified as ‘large artery atherosclerosis’ (27.5%), ‘cardioembolic’ (12.5%), ‘small artery occlusion’ (25.9%), ‘cryptogenic’ (31.5%) and ‘other’ (2.5%).21 Thus, we might expect a future R-TEST service to have considerably more referrals. This potential increase in R-TEST referrals was included in health economic modelling, which assumed R-TEST referral for all stroke or TIA patients who did not have known AF (n=415 over the one year study period).
Economic analysis
This study demonstrated a high likelihood of cost-effectiveness for the service. In addition to improving patient outcomes and quality of life, by reducing the risk of secondary stroke, implementation of R-TEST monitoring based on results of this 100-person study are predicted to lead to measurable clinical benefits for NHS Highland, including reduced bed days and fewer secondary stroke patients, as well as economic benefits in the form of net savings of up to £35,235.75 per year to the health board.
National context
The Royal College of Physicians National Clinical Guideline for Stroke 2016 recommends that, “people with ischaemic stroke or TIA who would be eligible for secondary prevention treatment for atrial fibrillation (anticoagulation or left atrial appendage device closure) should undergo a period of prolonged (at least 12 hours) cardiac monitoring” and “people with ischaemic stroke or TIA who would be eligible for secondary prevention treatment for atrial fibrillation and in whom no other cause of stroke has been found should be considered for more prolonged ECG monitoring (24 hours or longer), particularly if they have a pattern of cerebral ischaemia on brain imaging suggestive of cardioembolism”.2
Despite inclusion in national guidelines, and increasing evidence that asymptomatic AF post-stroke confers an increased risk of subsequent stroke, the provision of prolonged monitoring is not available in many centres. Prior to establishing the service in our centre, patients could not have prolonged monitoring and, at best, received a 24-hour monitor. It is highly likely that AF and pAF were missed. While we would need to perform a large-scale controlled trial to confirm this, a previous randomised-controlled trial found that, after a 14-day follow-up, 18% of post-ischaemic stroke patients that received standard practice plus seven days R-TEST monitoring were detected with pAF versus 2% in the standard practice control group.22 Nevertheless, there were several barriers to establishing this service at our hospital, including: a lack of suitable monitors; prolonged Holter monitoring being time-consuming for physiology staff. However, the availability of the R-TEST with automated analysis has greatly reduced the time taken to analyse recorded ECGs. The R-TEST analysis package for AF detection has been fully validated.23
Service implementation
Despite high-quality computer device analysis there were some challenges in establishing this service. Staff education, particularly on non-cardiology wards was important to ensure that devices were managed in an appropriate manner, including lead repositioning when displaced, and removal and replacement of devices during bathing. Therefore, establishing the service requires collaboration between departments and a willingness to deliver collaborative working to improve patient care. Despite these issues, after establishing R-TEST within the physiology department, the clinical service was relatively easy to implement.
For outpatients, the R-TEST was fitted either during the outpatient visit or remotely. In our region, due to geographical factors, we have established close working with general practitioners, and all remote (non-urban) general practitioners have a practice-based cardiac external loop recorder, either provided by the hospital or purchased locally. In the majority of cases, the general practitioner fits the monitor, with analysis and interpretation performed centrally in the physiology department. We have previously reported non-inferiority with this approach.24 This devolved service has proven of great worth with regard to patient convenience, reduced carbon footprint and, more recently, during the recent COVID-19 pandemic, where the focus has been to avoid unnecessary travel to the central hospital.
In the future we would expect that more patients would be referred, as under-referral was acknowledged as an issue in this service evaluation. Those developing new services should be cognisant of this and the likelihood of increased demand as clinicians become familiar with the new service.
Limitations
There are some limitations to our study: first, this was a single-centre study, but as it is the only stroke centre in our area, it is highly likely that the participants are representative of the general UK stroke population, and, therefore, it is likely that the results are generalisable. This was a small, short-term feasibility study, and we, therefore, did not report recurrent strokes or longer-term outcomes. However, other larger studies are ongoing to address these questions. We did not report detailed morbidity data in our cohort or absolute stroke risk based on CHA2DS2VASc score, and all patients had suffered a stroke or TIA and all were eligible for anticoagulation if AF was detected.
Key messages
- Routine post-stroke arrhythmia monitoring using R-TEST is feasible, effective at detecting atrial fibrillation (AF), and represents a probable clinical and economic benefit
- Staff education is key to ensuring appropriate patients are referred
Conflicts of interest
None declared.
Funding
This project was undertaken as part of a joint working agreement with Daiichi Sankyo Ltd. who funded the R-TEST monitors only. Daiichi Sankyo had no input over the study design, participant recruitment, data collection and analysis, or content of this report and Daiichi Sankyo did not write the report. AG is funded by Inverness and Highland City Region Deal.
Acknowledgement
The authors would like to thank the staff of the physiology department and stroke ward for their support while establishing this service.
References
1. Feigin VL, Forouzanfar MH, Krishnamurthi R et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–54. https://doi.org/10.1016/S0140-6736(13)61953-4
2. Intercollegiate Stroke Working Party. Stroke guidelines. London: Royal College of Physicians, 2016. Available from: https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines [accessed 28 October 2021].
3. Seiffge DJ, Werring DJ, Paciaroni M et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol 2019;18:117–26. https://doi.org/10.1016/S1474-4422(18)30356-9
4. Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atheroscler Rep 2006;8:310–16. https://doi.org/10.1007/s11883-006-0009-9
5. Kishore A, Vail A, Majid A et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack. Stroke 2014;45:520–6. https://doi.org/10.1161/STROKEAHA.113.003433
6. Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14:377–87. https://doi.org/10.1016/S1474-4422(15)70027-X
7. Krahn AD, Manfreda J, Tate RB, Mathewson FAL, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995;98:476–84. https://doi.org/10.1016/S0002-9343(99)80348-9
8. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. https://doi.org/10.1161/01.STR.22.8.983
9. EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255–62. https://doi.org/10.1016/0140-6736(93)92358-Z
10. Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease. Stroke 1983;14:537–40. https://doi.org/10.1161/01.STR.14.4.537
11. Ezekowitz MD, Bridgers SL, James KE et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. N Engl J Med 1992;327:1406–12. https://doi.org/10.1056/NEJM199211123272002
12. Klijn CJ, Paciaroni M, Berge E et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019;4:198–223. https://doi.org/10.1177/2396987319841187
13. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J 2010;31:967–75. https://doi.org/10.1093/eurheartj/ehn599
14. Banerjee A, Taillandier S, Olesen JB et al. Pattern of atrial fibrillation and risk of outcomes: the Loire Valley Atrial Fibrillation Project. Int J Cardiol 2013;167:2682–7. https://doi.org/10.1016/j.ijcard.2012.06.118
15. Gladstone DJ, Spring M, Dorian P et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. https://doi.org/10.1056/NEJMoa1311376
16. Sanna T, Diener H-C, Passman RS et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. https://doi.org/10.1056/NEJMoa1313600
17. Schnabel RB, Haeusler KG, Healey JS et al. Searching for atrial fibrillation poststroke. Circulation 2019;140:1834–50. https://doi.org/10.1161/CIRCULATIONAHA.119.040267
18. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. https://doi.org/10.1056/NEJMoa1310907
19. Saxena R, Koudstaal PJ. Anticoagulants for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev 2004;(2):CD000185. https://doi.org/10.1002/14651858.CD000185.pub2
20. Kishore AK, Fletcher S, Mason D, Ashton C, Molloy J, Fitchet A. Quality improvement in atrial fibrillation detection after ischaemic stroke (QUIT-AF). Clin Med 2020;20:480–5. https://doi.org/10.7861/clinmed.2020-0322
21. Haeusler KG, Kirchhof P, Kunze C et al. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol 2021;20:426–36. https://doi.org/10.1016/S1474-4422(21)00067-3
22. Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke 2013;44:2525–31. https://doi.org/10.1161/STROKEAHA.113.001927
23. Duverney D, Gaspoz J-M, Pichot V et al. High accuracy of automatic detection of atrial fibrillation using wavelet transform of heart rate intervals. Pacing Clin Electrophysiol 2002;25(4 Pt 1):457–62. https://doi.org/10.1046/j.1460-9592.2002.00457.x
24. Callum KJ, Hall L, Jack S, Farman C, Rushworth GF, Leslie SJ. External loop recorders: primary care placement is noninferior to hospital-based cardiac unit. J Prim Care Community Health 2020;11:2150132720946147. https://doi.org/10.1177/2150132720946147
