We report the case of a 45-year-old man presenting with worsening shortness of breath and chest tightness on a background of type 2 diabetes mellitus, hypertension and stable angina. He felt generally unwell and had a productive cough two weeks prior to presentation. Initial examination found quiet heart sounds and reduced air entry bi-basally on auscultation. Electrocardiography (ECG) demonstrating lateral T-wave flattening and ongoing chest tightness directed management towards an acute coronary syndrome (ACS). However, negative troponin I and positive D-dimer prompted investigation with computed tomography pulmonary angiogram (CTPA) identifying a 3.5 cm thickness pericardial effusion and no pulmonary embolism. Initial COVID-19 nasopharyngeal swabs were negative for SARS-CoV-2. Echocardiography identified features consistent with cardiac tamponade prompting pericardiocentesis. Over 1,000 ml of straw-coloured aspirate was drained with significant clinical improvement, and the patient was discharged with plans for urgent outpatient cardiac magnetic resonance imaging (MRI). Interestingly, despite multiple negative nasopharyngeal swabs for COVID-19, serum antibodies to SARS-CoV-2 were detected.
Case presentation
A 45-year-old man presented to the emergency department with a 10-day history of feeling unwell, non-exertional chest tightness, shortness of breath and reduced exercise tolerance. He found no relief with sublingual glyceryl trinitrate and complained of a productive, green cough. A COVID-19 nasopharyngeal assay prior to admission was negative for SARS-CoV-2. Past medical history included type 2 diabetes mellitus, hypertension and stable angina. There was no significant history of tobacco, alcohol or illicit substance use. On examination the patient was haemodynamically stable with positive findings of quiet heart sounds and reduced bi-basal air entry. The admission electrocardiogram (ECG) found new changes of lateral T-wave inversion (figure 1), so empirical treatment for acute coronary syndrome (ACS) was commenced. Admission biochemistry of note was raised inflammatory markers, a raised D-dimer and a normal high-sensitivity troponin I.

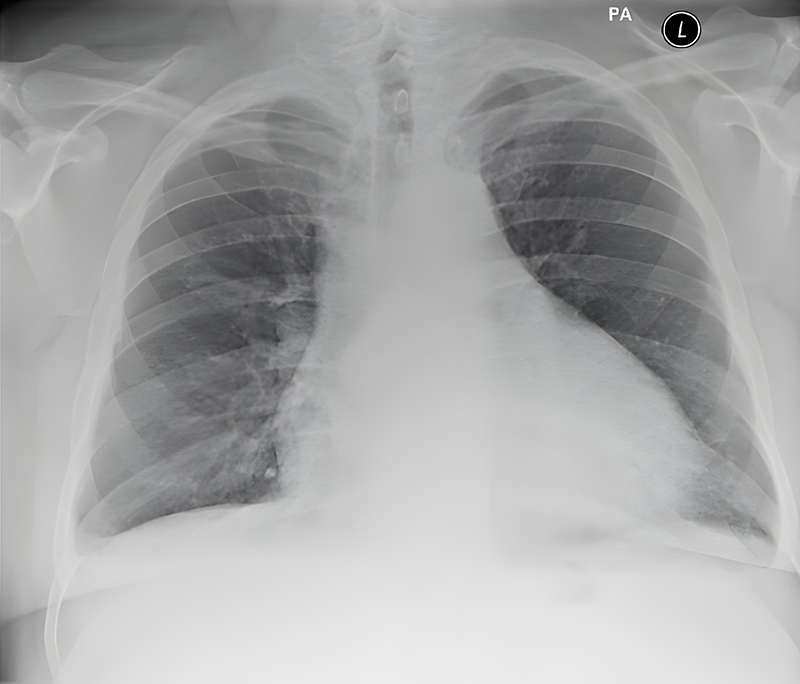
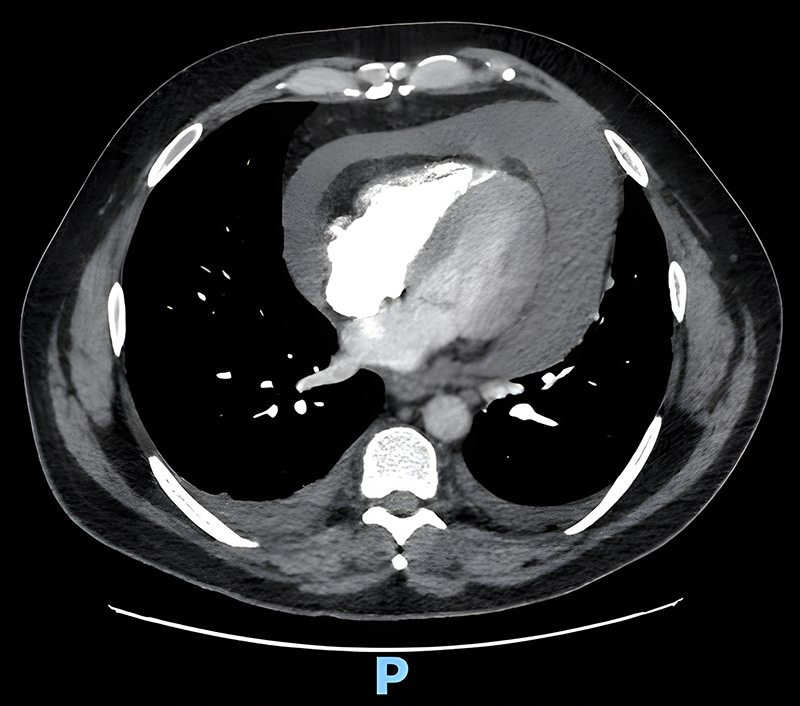
Further investigations included a repeat troponin (negative), chest X-ray (figure 2) and computed tomography pulmonary angiogram (CTPA), which demonstrated a 3.5 cm thickness pericardial effusion and small bilateral atelectasis/pleural effusions (figure 3). Microbiological investigations were negative for COVID-19: polymerase chain reaction (PCR), urine microscopy and cultures, and blood cultures. Differential diagnoses shifted from thromboembolic event to a constrictive/peri-myocarditis picture.
Intravenous antibiotics were commenced to cover for a respiratory infection (community-acquired pneumonia), and colchicine twice daily initiated for symptomatic pericarditis. However, the patient continued to deteriorate and became tachypnoeic and pyrexial (39.2°C). Repeat COVID-19 swab and blood cultures were negative.
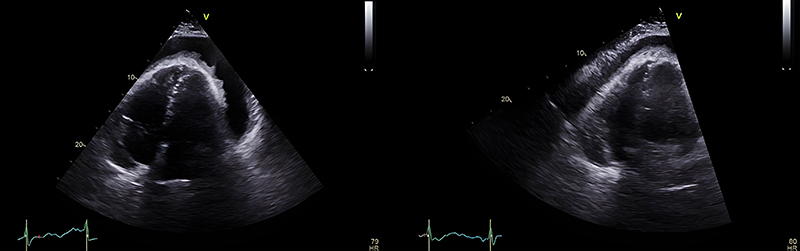
Echocardiography revealed a large global effusion with right atrial systolic collapse, right ventricular diastolic collapse and mitral valve inflow variation – features consistent with cardiac tamponade (figure 4). The patient underwent urgent pericardiocentesis in the catheter lab and a large volume of straw-coloured fluid was aspirated (and sent for analysis). A drain was left in situ with a plan for removal in 24–72 hours or until dry. Repeat echocardiogram 24 hours later showed a 1.2 cm organised effusion and approximately 1,100 ml of fluid had been aspirated. At this point, the patient had been afebrile for >48 hours and felt better symptomatically.
Unfortunately, pericardial aspirate sent for biochemistry and further analysis was unable to be carried out due to COVID-19-related laboratory restrictions. However, microbiological assessment found pus cells, with no organisms seen on Gram stain or cultures.
Investigations to rule out other causes of pericarditis included; a negative vascular work-up (rheumatoid factor, connective tissue disorder screen, cyclic citrullinated peptide [CCP] antibodies, antineutrophil cytoplasmic antibodies [cANCA]), a negative hepatitis serology, a negative antistreptolysin O test, erythrocyte sedimentation rate (ESR) (raised at 86 mm/hr), and a raised immunoglobulin (Ig)A (5.36 g/L; reference range 0.40–3.50 g/L) with normal IgG/IgM. Interestingly, despite multiple negative COVID-19 swabs, the SARS-CoV-2 antibody was positive on biochemical analysis.
Discussion
In March 2020 the World Health Organisation officially declared COVID-19 a global pandemic, with the index case thought to be as early on as early December 2019.1 Caused by the pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it rapidly disseminated across the world from its point of origin in Wuhan, China. Primarily compromising the respiratory system, COVID-19 presents with a spectrum of symptoms from entirely asymptomatic to acute respiratory distress syndrome (ARDS). Interestingly, and pertinent to this case report, COVID-19 has many extra-pulmonary presentations and complications, and we, as clinicians, need to be aware of them. The pathophysiology of COVID-19 can be grossly simplified into fusion between virus and cell membrane via binding at the angiotensin-converting enzyme 2 (ACE2) receptor, expressed in lung tissue, as well as other organs.2 The pathophysiology linking COVID-19 infection and myocarditis can be explained by the expression of ACE2 by cardiac myocytes and entry into the cell by binding the spike protein to the receptor.3 Epidemiological data on COVID-19 and cardiac injury comes with a particular limitation – the process of diagnosing myopericarditis. However, early data found around 7% incidence of myopericarditis and an increased mortality in patients with COVID-19 and associated cardiac injury.4
The application of the abovementioned principles may provide an explanation for this case report, however, despite the knowledge of extra-pulmonary manifestations of COVID-19, there is little in the literature about cardiac tamponade as a life-threatening complication.
There are several causes of cardiac tamponade, with the most common being pericarditis, malignant, iatrogenic or idiopathic.5 Infectious diseases account for the most common cause of cardiac tamponade.6 Diagnosis of cardiac tamponade is clinical (Beck’s triad is often implemented – quiet heart sounds, raised jugular venous pressure and hypotension), but ECG can also be used (ECG may show widespread low voltages) and/or radiology (a simple film of the chest may show an enlarged mediastinum and a globular cardiac silhouette). The echocardiograph can aid in diagnosis and management due to its quick, non-invasive nature. The European Society of Cardiology (ESC) have described some observational features of the heart and pericardium during cardiac tamponade.5
Early identification of cardiac tamponade is imperative, and management time critical, to prevent complete loss of cardiac output. At the point of discovery, intervention is primary, following with aetiological assessment. Pericardiocentesis is the definitive management and often patients require pre- and post-procedure monitoring in a high-dependency/intensive-care setting. Image-guided drainage of the effusion allows for safe and effective pericardiocentesis. Supportive management involves adequate oxygenation – this is of particular concern in a case such as this one. If the cause of the tamponade is SARS-CoV-2 then patients may also present with respiratory compromise requiring mechanical ventilation – in cardiac tamponade this can have antagonistic effects to the physiological compensation methods employed by the body and can lead to significant haemodynamic instability.7
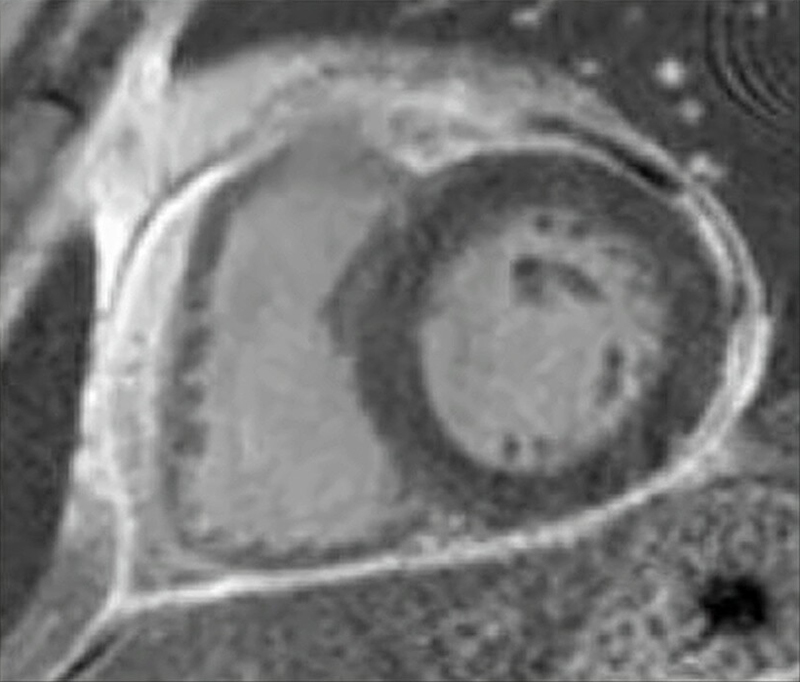
Unique to our case, following pericardiocentesis our patient went on to have further radiological assessment with magnetic resonance imaging (MRI) six weeks post-admission. Assessment of the pericardium found global pericardial enhancement with circumferential mild thickening of the pericardium measuring up to 4 mm, signs of oedema and late enhancement of the pericardium with no remaining pericardial effusion. However, a septal bounce was still present. As the repeat echocardiogram and cardiac MRI (figure 5) showed resolution of pericardial effusion, the patient was booked in for routine cardiology follow-up.
Key messages
- Acute pericarditis complicated by a pericardial effusion can be an uncommon presentation of COVID-19 and should be considered as it can be potentially life-threatening and is treatable with pericardiocentesis
- In patients presenting with a background of cardiac pathology or demonstrating cardiac pathology, imaging such as cardiac magnetic resonance or echocardiography is highly recommended
- Cardiac tamponade/pericarditis may present in a number of ways and it is important to recognise clinical and radiological features
- Diagnosis of COVID-19 is still in its rudimentary stage and one should be aware of the pitfalls of COVID-19 swab tests, i.e. swabs could be false negative while the antibody test is positive, as in our case
- Surrogate markers such as D-dimer, lymphocyte count and B-type naturietic peptide (BNP), although not diagnostic, may be clues to COVID infection, and one should have a high index of suspicion of COVID-related myocarditis/effusion in patients presenting with shortness of breath and/or chest pain
Conflicts of interest
None declared.
Funding
None.
Patient consent
Both verbal and written informed consent were obtained from the patient.
References
1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020;91:157–60. https://doi.org/10.23750/abm.v91i1.9397
2. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol 2020;215:108427. https://doi.org/10.1016/j.clim.2020.108427
3. Siripanthong B, Nazarian S, Muser D et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17:1463–71. https://doi.org/10.1016/j.hrthm.2020.05.001
4. Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Lane DA, Lip GYH. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Invest 2021;51:e13679. https://doi.org/10.1111/eci.13679
5. Jensen J, Poulsen S, Molgaard H. Cardiac tamponade: a clinical challenge. E-Journal of Cardiology Practice 2017;15. Available at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/Cardiac-tamponade-a-clinical-challenge
6. Dudzinski DM, Mak GS, Hung JW. Pericardial diseases. Curr Probl Cardiol 2012;37:75–118. https://doi.org/10.1016/j.cpcardiol.2011.10.002
7. Cooper JP, Oliver RM, Currie P, Walker JM, Swanton RH. How do the clinical findings in patients with pericardial effusions influence the success of aspiration? Br Heart J 1995;73:351–4. https://doi.org/10.1136/hrt.73.4.351
