There has been suggestion that vitamin D may play a role in protection against severe infection with COVID-19. In this article a potential mechanism involving angiotensin-converting enzyme 2 (ACE2) is proposed.
Introduction
Retrospective studies revealed that vitamin D may protect against severe COVID-19 disease,1,2 and some pilot studies suggest that it even improves prognosis.3,4 The two most widely accepted theories are the vitamin D modulation of immunity and the renin–angiotensin system.5,6 So far, the mechanism of the benefit of vitamin D in COVID-19 remains unknown.
Role of ACE2
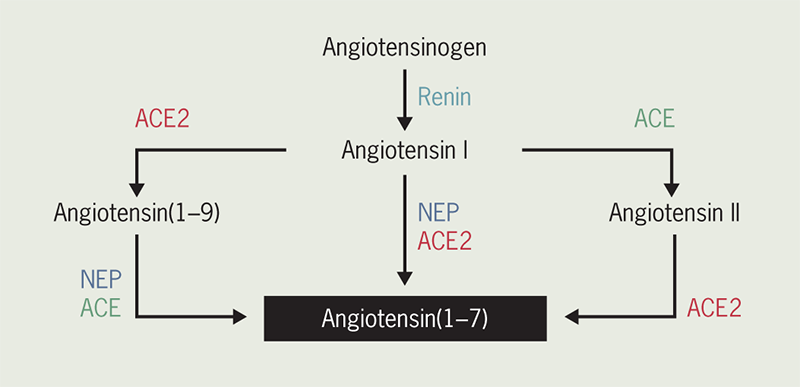
Angiotensin-converting enzyme 2 (ACE2) converts angiotensin (Ang) II to Ang(1–7) and Ang I to Ang(1–9) (figure 1).7 Ang(1–7) has a very short half-life (<9 seconds), and the release of a soluble catalytic ectodomain of ACE2 (ceACE2) from the vascular endothelium may serve to alter systemic Ang(1–7) concentrations and the relative peripheral balance of ACE2/ACE.8 Ang(1–7) acts on the Mas receptor to provide beneficial cardiovascular effects. Thus, the ACE2/Ang(1–7)/Mas axis exerts cardiovascular protection by providing antifibrotic, antihypertrophic, antithrombotic, and vasodilator effects. In contrast, the ACE/Ang II/Ang II receptor axis exerts the opposite effects.9 Many studies revealed that high levels of soluble ceACE2 plasma activity is a predictor of adverse cardiovascular events, cardiovascular mortality, and all-cause mortality,10,11 and also could be a potential biomarker of cardiac remodelling.11
Role of vitamin D
ACE2 can be present in two forms: the first as a transmembrane cell-associated form of ACE2, with mRNA cell expression present in the cardiovascular system, kidney, intestine, and alveolar type II cells,7,12 and the second as a soluble ceACE2 that is present in plasma and other body fluids.11,13 The catalytic active ectodomain of ACE2 is located in the extracellular region and undergoes shedding that results in a soluble ceACE2 form;11,13 this process is via a disintegrin and metalloproteinase domain-containing protein 17 (ADAM17),14 which is a protease up-regulated in heart failure (HF).8 In addition, the calcium signalling pathway is involved in the ceACE2 shedding process regulated by calmodulin (CaM),13 which is a ubiquitous calcium-binding protein. Basic science studies show that 1,25(OH)2D (vitamin D) enhances CaM function by increasing the ability of the vesicles to accumulate calcium.15 Moreover, a preclinical study proved that vitamin D produced slight increases in levels of soluble ceACE2 plasma activity.16
Implications for COVID-19
Many studies have proved that the administration of transgenic forms of soluble ceACE2 exerts an effect on the ACE2/Ang(1–7) axis modulation in HF, cardiovascular disease, and lung injury.9,17,18 Given that the catalytic ectodomain of ACE2 is an essential entry receptor for SARS-CoV2 infection, in vitro studies demonstrated that the administration of transgenic forms of soluble ceACE2 inhibits cell entry and replication of SARS-COV-2.19,20 Therefore, the administration of transgenic forms of soluble ceACE2, or enhancing the shedding of soluble ceACE2 with vitamin D, could be a potential therapy for inhibiting cell entry and replication of SARS-COV-2. Furthermore, vitamin D receptor is highly expressed in the cuboidal alveolar type II cells of the lung and preclinical studies revealed that overexpression of vitamin D receptor exerts anti-inflammatory effects in the lung, which decreases the storm of cytokines and chemokines.21 In this regard, meta-analysis (n=75541) reported that vitamin D supplementation was safe and had a small risk reduction of acute respiratory infections compared with placebo, protection was associated with administration of daily doses of 400–1000 IU for up 12 months and patients with severe vitamin D deficiency may experience the greatest benefit.22 Of note, to correct vitamin D deficiency in severely sick patients requires higher doses than usual, probably related to impaired hepatic conversion of vitamin D into 25-dihydroxyvitamin D.23 Thus, calcifediol may have some advantages over the native vitamin D, as it has a more reliable intestinal absorption (close to 100%) and can rapidly restore serum concentrations of 25-dihydroxyvitamin D, as it does not require hepatic 25-hydroxylation. Moreover, impaired hepatic 25-hydroxylation due to affected CYP2R1 expression has been demonstrated in preclinical models of obesity, diabetes and glucocorticoid excess.23 In this regard, some studies have showed that the administration of calcifediol (25-hydroxyvitamin D3) helps in the speedy recovery from early-stage mild to moderate symptoms of COVID-19,24 as well as reduced need for ICU treatment of patients requiring hospitalisation due to proven COVID-19.25 In addition, the studies demonstrated that the administration of calcifediol at high doses (0.532 mg on day 1, 0.266 mg on day 3 and 7, and then weekly until discharge) was well tolerated and without significant adverse effects.25
Hence, vitamin D replacement is a feasible and harmless adjuvant treatment for COVID-19, especially in those with vitamin D deficiency. Nevertheless, more clinical trials are required to confirm the therapeutic role of vitamin D in COVID-19.
Key messages
- Retrospective studies suggest that vitamin D may protect against severe COVID-19 disease
- The catalytic ectodomain (ce) of angiotensin-converting enzyme 2 (ACE2) is an essential entry receptor for SARS-CoV2 infection
- ACE2 can be present in two forms: a transmembrane cell-associated form of ACE2 and as a soluble ceACE2 that is present in plasma and other body fluids
- Vitamin D can enhance the shedding of soluble ceACE2 via the calmodulin calcium-signalling pathway
- Vitamin D could be a potential therapy for inhibiting cell entry and replication of SARS-COV-2
Conflicts of interest
None declared.
Funding
None.
References
1. Angelidi AM, Belanger MJ, Lorinsky MK et al. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc 2021;96:875–86. https://doi.org/10.1016/j.mayocp.2021.01.001
2. Infante M, Buoso A, Pieri M et al. Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: an Italian retrospective study. J Am Coll Nutr 2021;41:250–65. https://doi.org/10.1080/07315724.2021.1877580
3. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol 2020;203:105751. https://doi.org/10.1016/j.jsbmb.2020.105751
4. Annweiler G, Corvaisier M, Gautier J et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients 2020;12:3377. https://doi.org/10.3390/nu12113377
5. Aygun H. Vitamin D can reduce severity in COVID-19 through regulation of PD-L1. Naunyn Schmiedebergs Arch Pharmacol 2022;395:487–94. https://doi.org/10.1007/s00210-022-02210-w
6. Ahmed F. A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Front Immunol 2020;11:590459. https://doi.org/10.3389/fimmu.2020.590459
7. Donoghue M, Hsieh F, Baronas E et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1–E9. https://doi.org/10.1161/01.RES.87.5.e1
8. Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 2008;52:750–4. https://doi.org/10.1016/j.jacc.2008.02.088
9. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 2016;118:1313–26. https://doi.org/10.1161/CIRCRESAHA.116.307708
10. Narula S, Yusuf S, Chong M et al. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet 2020;396:968–76. https://doi.org/10.1016/S0140-6736(20)31964-4
11. García-Escobar A, Jiménez-Valero S, Galeote G, Jurado-Román A, García-Rodríguez J, Moreno R. The soluble catalytic ectodomain of ACE2 a biomarker of cardiac remodelling: new insights for heart failure and COVID19. Heart Fail Rev 2021;26:961–71. https://doi.org/10.1007/s10741-020-10066-6
12. Qian Z, Travanty EA, Oko L et al. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol 2013;48:742–8. https://doi.org/10.1165/rcmb.2012-0339OC
13. García-Escobar A, Vera-Vera S, Jurado-Román A, Jiménez-Valero S, Galeote G, Moreno R. Calcium signaling pathway is involved in the shedding of ACE2 catalytic ectodomain: new insights for clinical and therapeutic applications of ACE2 for COVID-19. Biomolecules 2022;12:76. https://doi.org/10.3390/biom12010076
14. Iwata M, Silva Enciso JE, Greenberg BH. Selective and specific regulation of ectodomain shedding of angiotensin-converting enzyme 2 by tumor necrosis factor alpha-converting enzyme. Am J Physiol Cell Physiol 2009;297:C1318–C1329. https://doi.org/10.1152/ajpcell.00036.2009
15. Bikle DD, Munson S. 1,25-Dihydroxyvitamin D increases calmodulin binding to specific proteins in the chick duodenal brush border membrane. J Clin Invest 1985;76:2312–16. https://doi.org/10.1172/JCI112241
16. Bártová E, Legartová S, Krejčí J, Arcidiacono OA. Cell differentiation and aging accompanied by depletion of the ACE2 protein. Aging (Albany NY) 2020;12:22495–508. https://doi.org/10.18632/aging.202221
17. Khan A, Benthin C, Zeno B et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 2017;21:234. https://doi.org/10.1186/s13054-017-1823-x
18. Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol 2017;69:805–19. https://doi.org/10.1016/j.jacc.2016.11.064
19. Monteil V, Kwon H, Prado P et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020;181:905.e7–913.e7. https://doi.org/10.1016/j.cell.2020.04.004
20. Tada T, Fan C, Chen JS et al. An ACE2 microbody containing a single immunoglobulin Fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep 2020;33:108528. https://doi.org/10.1016/j.celrep.2020.108528
21. Ishii M, Yamaguchi Y, Isumi K et al. Transgenic Mice Overexpressing Vitamin D Receptor (VDR) Show Anti-Inflammatory Effects in Lung Tissues. Inflammation 2017;40:2012–19. https://doi.org/10.1007/s10753-017-0641-2
22. Jolliffe DA, Camargo CA Jr, Sluyter JD et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol 2021;9:276–292. https://doi.org/10.1016/S2213-8587(21)00051-6
23. Bouillon R, Bikle D. Vitamin D Metabolism Revised: Fall of Dogmas. J Bone Miner Res 2019;34:1985–1992. https://doi.org/10.1002/jbmr.3884
24. Khan A, Iqtadar S, Mumtaz SU et al. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial. Front Pharmacol 2022;13:898062. https://doi.org/10.3389/fphar.2022.898062
25. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM et al. “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”. J Steroid Biochem Mol Biol 2020;203:105751. https://doi.org/10.1016/j.jsbmb.2020.105751
