The National Institute for Health and Care Excellence (NICE) advise against routine testing for coronary artery disease (CAD) in patients with non-anginal chest pain (NACP). This clinical audit sought to establish the prevalence of significant CAD in this cohort using computed tomography angiography (CTCA) and evaluate differences in the prevalence of cardiovascular risk factors between those with and without obstructive coronary disease.
Over 23 months, 866 patients with NACP underwent CTCA. Patients were separated into three groups for analysis depending on the degree of CAD on CTCA using the CAD-RADS (Coronary Artery Disease Reporting and Data System) scoring system; no evidence of CAD (group 1), a degree of CAD requiring medical therapy only (group 2), significant CAD defined as a CAD-RADS score 4A/B or 5 (group 3). Cardiovascular risk factors were compared between the groups.
We found 11.5% had significant CAD (group 3), 58.3% required medical therapy (group 2) and 30.1% had no CAD (group 1). There were 32 patients who required coronary revascularisation. Patients in group 2 and 3 were more likely to be male (p<0.001) and older (p<0.001) when compared to patients in group 1. Patients in group 3 were more likely to be hypertensive (p=0.008) and have higher Qrisk2 scores (p<0.001) when compared with those in group 1.
In conclusion, NICE guidelines for NACP may result in a significant proportion of patients with CAD being underdiagnosed, including some with severe disease requiring revascularisation. This analysis suggests age, male gender, Qrisk2 score and hypertension are predictors of CAD in this cohort.
Introduction
Coronary artery disease (CAD) is a significant cause of morbidity and mortality in the UK, and anginal chest pain is the most common manifestation.1,2 Chest pain, however, is one of the most common presenting symptoms in both emergency, primary and secondary care, and can be secondary to a myriad of pathologies. Therefore, the diagnosis of CAD from symptoms alone can prove challenging.
Despite developments in both invasive and non-invasive CAD imaging, these investigations are not without risks and cost to the health service. Reduction of unnecessary investigations, while identifying patients most at risk, has been a major motivation behind clinical guidelines. While the latest European and American guidelines have adopted risk-based strategies to decide who to test for CAD,3,4 the National Institute for Health and Care Excellence (NICE) clinical guidance published in 2016 has recommended the use of patient symptoms alone.5 The three key characteristics of anginal chest pain are: the location and character of the pain; whether or not the pain is precipitated by physical exertion; and whether or not the pain can be relieved within five minutes by rest or glyceryl trinitrate spray.5 Patients who only have one of these three key features are said to have non-anginal chest pain (NACP), and the NICE guidance is to abandon routine testing for this cohort.
While recognised as a valid tool for predicting the likelihood of obstructive CAD,6,7 the use of symptoms alone can prove troublesome, as patients presenting with typical angina only account for 10–15% of patients with CAD.8–10 This can be particularly problematic when assessing female patients, who are more likely to present with atypical chest pain symptoms compared to males.11 Studies suggest that the majority of CAD cases present to cardiology clinics with NACP or atypical angina.10 Furthermore, there is some evidence to suggest patients presenting with NACP are, in fact, more likely to have underlying CAD than those with atypical angina.9 This, combined with a tendency for clinicians to under-recognise anginal symptoms, means that current NICE guidelines may result in a significant proportion of patients with CAD being underdiagnosed, inappropriately reassured, and discharged without investigations.12
Despite the 2016 recommendations, the rapid access chest pain clinic (RACPC) in our institution continued to investigate patients with NACP based on a local cardiac network agreement. Using data from the clinic, this clinical audit aimed to establish the prevalence of obstructive CAD in this cohort, and look for predictive markers that might help to risk stratify such patients in the future.
Materials and method
Between 1 January 2017 and 1 December 2019, 1,034 patients with no prior history of ischaemic heart disease presented to the RACPC with one of the three key features of typical angina (and were, therefore, deemed to have NACP), and were referred for computed tomography coronary angiography (CTCA). Patients that were unable to have CTCA due to poor renal or lung function were instead referred for stress echocardiography and were excluded from the analysis. All patients attending the clinic received information sheets explaining their anonymised data may be used for service evaluation and clinical audit. As the analysis was deemed to be a clinical audit, ethical approval from the trust’s local ethics committee was not required.
Patients were identified using the clinic database, and the trust’s electronic record system and imaging database were used to analyse CTCA results. During clinic triage, the incidence of cardiovascular risk factors was recorded and documented in the clinic database for each patient. These risk factors included age, gender, diabetes, hypertension, family history of cardiovascular disease and hypercholesterolaemia. The Qrisk2 score was also calculated for all patients.13
All patients were referred for a CTCA as a first-line non-invasive investigation for CAD, and their images were read by expert readers. The CAD-RADS (Coronary Artery Disease Reporting and Data System) scoring system was used to establish the degree of luminal stenosis and the coronary calcium score (Agatston/volume) was used to establish the calcium burden on CTCA.14 For the purpose of this analysis, patients were divided into three groups according to the degree of CAD on CTCA:
- Group 1 (no evidence of CAD): CAD-RADS score 0
- Group 2 (degree of CAD requiring medical preventative therapy only): CAD-RADS score 1–3
- Group 3 (significant CAD): CAD-RADS 4A/4B or 5.
Within group 2, the number of patients with a Qrisk2 score of less than 10% was also calculated, as this cohort would not have been eligible for a primary prevention statin prior to investigations, according to NICE guidelines.15 Patients with a CAD-RADS score of 3, or severe calcification on CTCA, are deemed high risk and often undergo further testing for CAD. Therefore, along with those in group 3, the records of patients in group 2 or with severe calcification on CTCA, were also analysed to identify those that underwent invasive angiography and revascularisation procedures.
The prevalence of the aforementioned cardiovascular risk factors were compared between each group. The ANOVA test was used to compare age, the Kruskal-Wallis test to compare Qrisk2 scores and the Chi-squared test to compare gender and the prevalence of diabetes, hypertension, family history and hypercholesterolaemia between the groups. Where significant differences between groups were found, post-hoc analysis was used to account for any false discovery. Where significant differences were found between the groups, 95% confidence intervals (CI) were calculated.
Results
Table 1. Reasons for patients not having luminal analysis on computed tomography coronary angiography (CTCA)
| Reasons for non-interpretable CTCA | No. of patients |
|---|---|
| Severe calcification | 111 |
| Tachycardia | 34 |
| Poor image or motion artefact | 12 |
| Contrast allergy or side effects | 4 |
| Non-attendance or refused scan | 4 |
| Atrial fibrillation | 3 |
In total, 1,034 patients attended the clinic with NACP and were referred for a CTCA. Four patients refused to have the scan or did not attend, and four patients were unable to have the scan due to previous contrast allergies or side effects. Of the 1,026 patients who proceeded with a CTCA, 160 patients were unable to have luminal analysis due to severe calcification, gating difficulties (due to tachycardia or atrial fibrillation) and poor images or motion artefacts (see table 1 for breakdown).
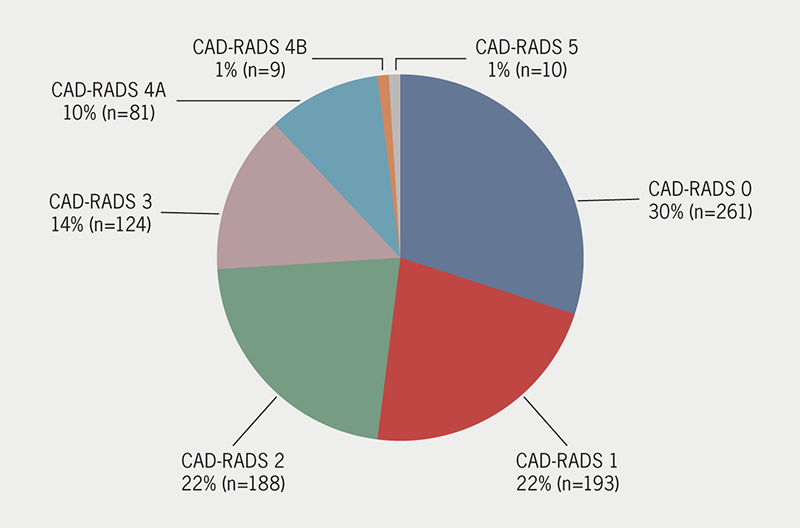
In total, 866 patients (51.4% male, 48.6% female) had a CTCA that allowed luminal analysis and calcium scoring. We found that 30.1% (n=261) of patients met the criteria for group 1 and had no evidence of CAD on their CTCA. A further 58.3% (n=505) of patients had a CAD-RADS score of 1–3 and required medical preventative therapy. Within this group, 32.5% (n=164) had a Qrisk2 score of less than 10%. In total, 11.5% (n=100) of patients met the criteria for group 3 and had evidence of significant CAD on CTCA. The CAD-RADS scores for these patients can be seen in figure 1. Nine patients from group 2, 37 patients from group 3, and 12 patients with severe calcification on CTCA underwent invasive angiography following their CTCA to add clarity to the diagnosis. In group 2, two patients underwent revascularisation procedures (one had a percutaneous coronary intervention [PCI] and one had a coronary artery bypass graft [CABG]). Six patients with severe calcification on their initial CTCA underwent revascularisation (two PCI and four CABG). In group 3, 24 patients underwent revascularisation procedures (19 PCI and five CABG).
With regards to cardiovascular risk factors, analysis revealed that patients in either group 2 or group 3 were more likely to be older than patients in group 1 (group 1 55.6 ± 10.3 years vs. group 2 60 ± 9.9 years, p<0.001; vs. group 3 62.3 ± 9.3 years, p<0.001). Furthermore, patients in group 2 and group 3 were more likely to be male compared to those in group 1 (55% in group 2 vs 40% in group 1, p<0.001; 62% in group 3 vs 40% in group 1, p<0.001). Patients with significant CAD were also more likely to be hypertensive than patients with no CAD on CTCA (28% in group 1 vs. 45% in group 3, p=0.008). Furthermore, significant differences in Qrisk2 scores were found between all three groups (figure 2). Patients in group 3 had a mean Qrisk2 score of 19.5 (interquartile range [IQR] 12.4–27), compared with 13.5 (IQR 8.3–20.4) in group 2 and 9.1 (IQR 6.4–14.9) in group 1. The differences in Qrisk2 scores between all three groups were significant at p<0.001. Although patients in group 3 were more likely to be diabetic and have a family history of cardiovascular disease when compared with the those in group 1 and 2, this was not statistically significant (p=0.217 and p=0.314, respectively). No significant difference in the prevalence of hypercholesterolaemia was found between the three groups (table 2).

Table 2. A comparison of the prevalence of cardiovascular risk factors between the three groups
| Variables | All patients (n=866) | Group one (n=261) | Group two (n=505) | Group three (n=100) | Test | p value | Post-hoc test | Differences between groups |
|---|---|---|---|---|---|---|---|---|
| Mean age ± SD, years | 58.9 ± 10.2 | 55.6 ± 10.3 | 60 ± 9.9 | 62.3 ± 9.3 | ANOVA | <0.001 | Tukey HSD | Group 1 significantly different to groups 2 and 3 |
| Male gender, n (%) | 445 (51) | 105 (40) | 278 (55) | 62 (62) | Chi-squared | <0.001 | Prop test | Group 1 significantly different to groups 2 and 3 |
| Qrisk2, median (IQR) | 12.7 (7.7–20.5) | 9.1 (6.4–14.9) | 13.5 (8.3–20.4) | 19.5 (12.4–27) | Kruskal-Wallis | <0.001 | Pair-wise Wilcoxon rank sum | All groups significantly different |
| Diabetes, n (%) | 117 (14) | 36 (14) | 61 (12) | 20 (20) | Chi-squared | 0.217 | ||
| Hypertension, n (%) | 293 (34) | 73 (28) | 175 (35) | 45 (45) | Chi-squared | 0.008 | Prop test | Group 1 significantly different to group 3 |
| Family history, n (%) | 354 (41) | 105 (40) | 202 (40) | 47 (47) | Chi-squared | 0.314 | ||
| Hypercholes-terolaemia, n (%) | 144 (17) | 39 (15) | 91 (18) | 14 (14) | Chi-squared | 0.856 | ||
| Key: ANOVA = analysis of variance; HSD = honestly significant difference; IQR = interquartile range; SD = standard deviation | ||||||||
Discussion
This analysis of patients presenting with NACP in a region of high population prevalence of CAD, showed that nearly two-thirds had some degree of coronary atheroma on CTCA. Indeed, 11.5% of patients had obstructive CAD on CTCA, with around a quarter of these patients having prognostic disease warranting revascularisation procedures. A further 58.3% had a degree of CAD warranting medical therapy with statins. Of these patients, 32.5% had a Qrisk2 score of less than 10% and, therefore, would not have qualified for a primary prevention statin prior to their CTCA, according to NICE guidelines. Patients with a CAD-RADS score of 3 at our institution went on to have functional testing, however, this audit did not capture those found to have ischaemia on these tests. This, along with our exclusion of patients with high calcium scores prohibiting luminal analysis, means the prevalence of significant CAD may be even higher in this cohort. Our clinical audit suggests that adhering to current NICE guidelines results in a significant proportion of patients with CAD being underdiagnosed and wrongly reassured.
The prevalence of severe CAD in this clinical audit is similar to other databases. Data from the international, multi-centre CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry revealed that 11.7% of 1,253 patients with NACP undergoing clinically indicated CTCA had >70% luminal stenosis.9 In their database, the actual prevalence of >50% luminal stenosis was in fact lower than guideline prediction for most patients presenting with chest pain. The only exception was the NACP cohort who, in fact, had higher than predicted moderate CAD on angiography. In the American College of Cardiology registry, patients with atypical angina had the lowest prevalence of CAD of all the groups, including those with NACP and no chest pain.16 As well as this, there is evidence that NACP is associated with significant morbidity and mortality if left untreated. In their five-year analysis of patients attending RACPCs across multiple centres in England, Sekhri et al. found that patients diagnosed with NACP accounted for around a third of patients that suffered either acute coronary syndrome or died from cardiovascular disease during follow-up.17 This suggests that NICE guidance proposing investigations for patients with atypical angina, and not for those with NACP, may be a missed opportunity.
With all guidance for suspected angina, there is a balance to be struck between the widespread use of a technology with a negative yield, yet identifying the patients at highest risk of needing revascularisation. If the purpose of the NICE guidance is to diagnose patients with obstructive CAD, then data from the CONFIRM and American registries suggest that the only group that should be investigated with anatomical testing is those with typical angina, as this is the only symptom group that strongly predicted significant obstructive disease on CTCA.9,16 However, in their post-hoc analysis of the 2015 SCOT-HEART (Scottish Computed Tomography of the Heart) study dataset,18 Adamson et al. found that CTCA imaging resulted in an increase in the rates of invasive angiography in patients with NACP, with no increase in the pick-up of obstructive disease and revascularisation procedures, thereby, supporting the use of NICE guidelines.19 Nevertheless, CTCA still identified obstructive disease in 9.5% of patients with NACP, with 2.2% of patients undergoing revascularisation procedures and 19.4% having treatment changes. In the initial 2015 SCOT-HEART study, the improved outcomes in patients undergoing CTCA versus standard care were achieved through preventative therapies rather than revascularisation,18 highlighting the importance of optimising medical therapy in this cohort. In our analysis, 18.9% of patients with NACP would not have benefited from appropriate preventative therapy under the current NICE guidance.
We do, however, acknowledge that the blanket use of imaging in all patients with NACP is not without its disadvantages. The obvious difficulties include cost, as well as the manpower to perform and interpret imaging. Although there is increasing evidence to suggest CTCA is a cost-effective approach to identify CAD in patients with low-to-intermediate risk of disease, very few studies include the ‘no-testing’ approach proposed by NICE.20 Furthermore, even if significant CAD is detected on anatomical imaging, there is little evidence to suggest that revascularisation of patients with stable CAD improves outcomes.21 This may suggest that, unless patients are limited by their angina symptoms and refractory to medical therapy, an increased detection of significant CAD on imaging may lead to unnecessary and high-risk procedures.22
In conclusion, this clinical audit suggests that current NICE guidelines for the investigation of patients with NACP may lead to the underdiagnosis of significant disease, especially in high prevalence regions of the UK.1 This analysis found that nearly two-thirds of the patients attending our centre had CAD even with chest pain that was classified as non-anginal. Qrisk2 score, male gender, age and a history of hypertension were the strongest predictors of CAD in this cohort. While RACPCs are not intended for population screening for CAD, our findings suggest that a more nuanced use of the NICE guidelines should be considered, especially in high prevalence areas.
Key messages
- Current National Institute for Health and Care Excellence (NICE) guidelines advise against routine testing for coronary artery disease (CAD) in patients presenting with non-anginal chest pain (NACP)
- Nearly two-thirds of patients attending a rapid access chest pain clinic with NACP had a degree of CAD on computed tomography coronary angiography (CTCA). A total of 11.5% of patients had significant CAD on CTCA, with around a quarter of these patients requiring revascularisation procedures
- Qrisk2 score, age, male gender and a history of hypertension were predictors of CAD in this cohort
- Our findings suggest that a more nuanced use of the NICE guidelines for NACP should be considered, especially in high prevalence areas
Conflicts of interest
None declared.
Funding
None.
Study approval
All patients attending the clinic received information sheets explaining their anonymised data may be used for service evaluation and clinical audit. As the analysis was deemed to be a clinical audit, ethical approval from the trust’s local ethics committee was not required.
References
1. British Heart Foundation. UK Factsheet January 2022. London: BHF, 2022. Available from: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics [accessed 25 November 2022].
2. Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study. Prognosis and survival. Am J Cardiol 1972;29:154–63. https://doi.org/10.1016/0002-9149(72)90624-8
3. Task Force Members, Montalescot G, Sechtem U et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. https://doi.org/10.1093/eurheartj/eht296
4. Gibbons RJ, Chatterjee K, Daley J et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. Circulation 1999;99:2829–48. https://doi.org/10.1161/01.CIR.99.21.2829
5. National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. CG95. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/cg95/chapter/Recommendations
6. Diamond G. A clinically relevant classification of chest discomfort. J Am Coll Cardiol 1983;1:574–5. https://doi.org/10.1016/S0735-1097(83)80093-X
7. Genders TS, Steyerberg EW, Hunink MG et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohort. BMJ 2012;344:e3485. https://doi.org/10.1136/bmj.e3485
8. Reeh J, Therming CB, Heitmann M et al. Prediction of coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J 2018;40:1426–35. https://doi.org/10.1093/eurheartj/ehy806
9. Cheng VY, Berman DS, Rozanski A et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation 2011;124:2423–32. https://doi.org/10.1161/CIRCULATIONAHA.111.039255
10. Douglas PS, Hoffmann U, Patel MR et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. https://doi.org/10.1056/NEJMoa1415516
11. Sharma SP, Manintveld OC, Buddle R et al. Gender Differences in Patients With Stable Chest Pain. Am J Cardiol 2022;171:84–90. https://doi.org/10.1016/j.amjcard.2022.01.054
12. Arnold SV, Grodzinsky A, Gosch KL et al. Predictors of physician under-recognition of angina in outpatients with stable coronary artery disease. Circ Cardiovasc Qual Outcomes 2016;9:554–9. https://doi.org/10.1161/CIRCOUTCOMES.116.002781
13. Collins GS, Altman DG. An independent and external validation of QRISK2 cardiovascular disease risk score: a prospective open cohort study. BMJ 2010;340:c2442. https://doi.org/10.1136/bmj.c2442
14. Cury RC, Abbara S, Achenbach S et al. CAD-RADS™: Coronary Artery Disease – Reporting and Data System: an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Am Coll Radiol 2016;13(12 Pt A):1458.e9–1466.e9. https://doi.org/10.1016/j.jacr.2016.04.024
15. National Institute for Health and Care Excellence. Cardiovascular risk assessment and lipid modification. QS100. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/qs100/chapter/quality-statement-5-statins-for-primary-prevention
16. Patel MR, Peterson ED, Dai D et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2014;362:886–95. https://doi.org/10.1056/NEJMoa0907272
17. Sekhri N, Feder GS, Junghans C et al. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 2007;93:458–63. https://doi.org/10.1136/hrt.2006.090894
18. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. https://doi.org/10.1016/S0140-6736(15)60291-4
19. Adamson PD, Hunter A, Williams MC. Diagnostic and prognostic benefits of computed tomography coronary angiography using the 2016 National Institute for Health and Care Excellence guidance within a randomised trial. Heart 2018;104:207–14. https://doi.org/10.1136/heartjnl-2017-311508
20. van Waardhuizen CN, Khanji MY, Genders TS et al. Comparative cost-effectiveness of non-invasive imaging tests in patients presenting with chronic stable chest pain with suspected coronary artery disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2016;2:245–60. https://doi.org/10.1093/ehjqcco/qcw029
21. Lin GA, Dudley RA. Fighting the “oculostenotic reflex”. JAMA Intern Med 2014;174:1621–2. https://doi.org/10.1001/jamainternmed.2014.164
22. Maron DJ, Hochman JS, Reynolds HR et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–407. https://doi.org/10.1056/NEJMoa1915922
