Our objective was to compare the efficacy of atrial fibrillation (AF) ablation versus permanent pacemaker (PPM) with atrioventricular node ablation (AVNA) versus direct current cardioversion (DCCV) for persistent AF in patients ≥65 years old.
Seventy-seven patients (aged 66–86, mean 75.4 years) with persistent AF were randomised (1:1:1) to AF ablation + amiodarone (± DCCV), PPM with AVNA (+DCCV) or DCCV + amiodarone. The primary end point was persistent AF recurrence, measured with an implanted cardiac monitor or PPM. Cardiopulmonary exercise testing (CPET) was performed at baseline and six months. Symptom questionnaires were completed monthly. Follow-up was 12 months.
The primary end point occurred in fewer patients following AF ablation + amiodarone than DCCV + amiodarone (seven patients, 28% vs. 15 patients, 60%; hazard ratio [HR] 0.559, 95% confidence interval [CI] 0.293 to 1.065, p=0.073) with no differences between DCCV + amiodarone and PPM with AVNA (HR 0.990, 95%CI 0.539 to 1.818, p=0.973). AF ablation + amiodarone resulted in a lower AF burden at 12 months compared with DCCV + amiodarone (17.0 ± 37.9% vs. 61.7 ± 48.6%, p<0.0001). Modified European Heart Rhythm Association (EHRA) symptom class improved in all patients (baseline 2.4 ± 0.495 vs. 12-month follow-up 1.84 ± 0.081, p=0.00001). Six-month CPET demonstrated a higher VO2 peak in sinus rhythm (SR) compared with baseline in AF (12.1 ± 4.2 ml/kg/min at baseline to 15.3 ± 4.2 ml/kg/min at six months, p=0.013).
In conclusion, in older patients with persistent AF, ablation + amiodarone resulted in a lower AF burden at 12 months than DCCV + amiodarone. There was a non-significant trend toward reduced recurrence of device-detected persistent AF episodes. All therapies improved symptoms despite DCCV restoring SR in <50% of patients at 12 months. CPET demonstrated improved VO2 peak with SR restoration.
Introduction
The optimal treatment for persistent atrial fibrillation (AF) in patients ≥65 years is unknown. There are several options including medical therapy for rate and rhythm control, direct current cardioversion (DCCV), permanent pacemaker (PPM) and atrioventricular node ablation (AVNA) and catheter ablation of AF.1 These treatment options have not been directly compared and each has its own advantages and disadvantages.2 In many patients, it is desirable to attempt to restore and maintain sinus rhythm to reduce symptoms and improve quality of life (QoL).3
DCCV has been reported to terminate AF in ≥90% of cases.4 However, recurrences are common with only 30–40% of patients remaining in sinus rhythm (SR) after one year without the use of adjuvant anti-arrhythmic drugs.5 PPM and AVNA improves QoL and symptoms when the rate cannot be controlled by pharmacological agents and, in the absence of underlying heart disease, survival has been reported to be similar to the general population.1,6,7 Patients are reliant on the PPM, thereafter, to provide a physiological heart rate. Catheter ablation of AF is a recommended therapeutic option in paroxysmal AF, but its overall efficacy and clinical benefit in patients with persistent AF ≥65 years has been repeatedly challenged.8
This is the first trial to compare DCCV versus AF ablation versus PPM and AVNA utilising continuous monitoring with implanted cardiac monitors (ICMs). In addition, we compared patient symptom questionnaires and cardiopulmonary exercise testing (CPET), and followed all patients for a minimum of 12 months.
Method
Study population and protocol
This prospective randomised study was conducted in a single arrhythmia referral centre between June 2016 and July 2018. Patients ≥65 years with symptomatic persistent AF were randomised 1:1:1 to DCCV + amiodarone, PPM and AVNA (+DCCV), and AF catheter ablation + amiodarone (±DCCV). The study protocol was approved by the institutional review board and national ethics committee (ClinicalTrials.gov identifier #NCT02528604, REC reference 16/LO/0727, IRAS project ID 195936). All patients gave written informed consent.
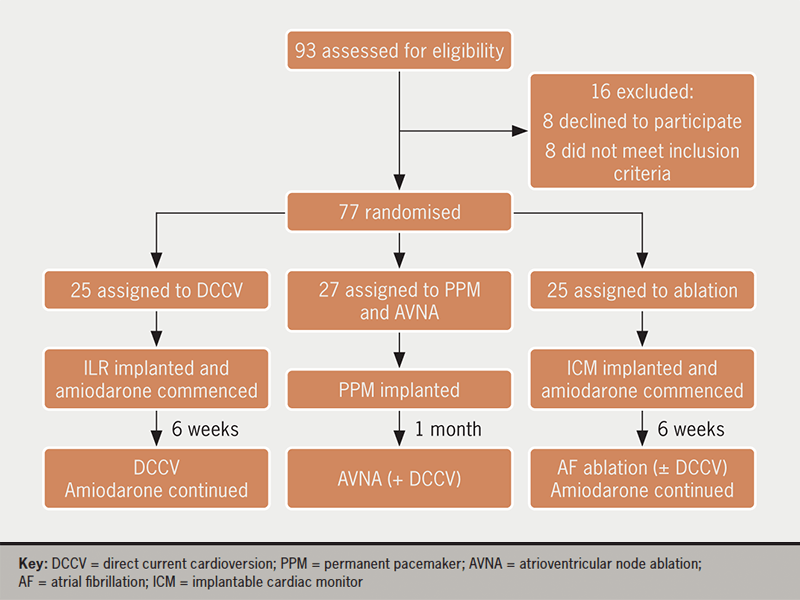
Inclusion criteria were age ≥65 years and symptomatic persistent AF. Exclusion criteria were: paroxysmal AF, long-standing persistent or permanent AF (defined according to Heart Rhythm Society [HRS] guidelines9), pregnancy, cardiac rhythm disorders other than AF, and the presence of pre-existing PPMs or ICMs. All patients had continuous arrhythmia monitoring with implanted devices for at least one month before and one year after intervention (figure 1) enabling objective assessment of AF burden. Patients underwent device downloads at three, six, nine and 12 months, as per the HRS expert consensus statement guidelines for catheter ablation trials.9
DCCV
Patients were implanted with a Medtronic Reveal XT (Minneapolis, MN, USA) ICM according to manufacturers’ instructions. Amiodarone was commenced, loaded, and monitored according to British National Formulary (BNF) instructions.
After six weeks of amiodarone therapy, patients underwent DCCV (provided the patient had remained in AF), and this was repeated if AF was found to have recurred during follow-up. Amiodarone therapy was continued throughout the study, unless the patient developed unacceptable side effects to the medication. The DCCV protocol was as per the local guidelines at our institution, described previously.10 All patients were therapeutically anticoagulated for at least three weeks pre-procedure, and anticoagulation was continued post-procedure.
PPM and AVNA
Patients underwent implantation of a standard dual-chamber PPM or biventricular device according to European Society of Cardiology (ESC) guidelines.3 A routine PPM check was performed one month post-implant to ensure device and lead stability. Following this, AVNA was performed. Complete heart block was achieved by permanently destroying the AV node or His bundle utilising percutaneous catheter radiofrequency ablation under conscious sedation. At the end of the procedure, patients underwent DCCV to restore sinus rhythm, as per local guidelines.10 The atrial lead allowed continuous assessment of atrial rhythm throughout the study.
Catheter ablation of AF
Patients initially underwent implantation of a Medtronic Reveal XT ICM and were simultaneously commenced on amiodarone (loading, maintenance, and monitoring of this medication as per BNF instructions).
After six weeks of amiodarone therapy, patients underwent left atrial ablation using a 28 mm cryoballoon to achieve pulmonary vein isolation. The procedures, which comprised pulmonary vein isolation with no additional substrate modification, were performed under conscious sedation. A decapolar catheter was positioned in the coronary sinus as a reference and for pacing. A single trans-septal puncture was performed under fluoroscopic guidance. A 14-F deflectable sheath (FlexCath, Medtronic, Minneapolis, MN, USA) was advanced through the trans-septal puncture. Cryoenergy was delivered using a 28 mm balloon (second-generation Arctic Front Advance, Medtronic). The balloon was advanced to the ostium of each pulmonary vein (PV). Ablation of PV antra was performed. Continuous monitoring of the phrenic nerve during ablation of the right PVs was systematically performed by pacing the right phrenic nerve with the decapolar catheter in the superior vena cava or right subclavian vein.
If a patient remained in AF at the end of their procedure, DCCV was performed to achieve sinus rhythm. A three-month blanking period was observed as per HRS guidelines for catheter ablation.11 If AF recurred within three months, a further DCCV was performed. If AF recurred within the first 12-month period, a repeat ablation utilising technology at the operator’s discretion was offered. Amiodarone therapy was continued throughout the duration of the study, unless the patient developed unacceptable side effects.
Continuous arrhythmia monitoring
AF was detected using continuous monitoring, either with a Medtronic Reveal XT ICM (DCCV and AF ablation arms) or PPM Holter (PPM and AVNA). The use of these modalities enabled an assessment of AF burden in all of the treatment arms.
Cardiopulmonary exercise testing
CPET was performed one week before and six months post-randomised AF intervention. A semi-recumbent tilting cycle ergometer (ERG 911 S/L, Schiller, Baar, Switzerland) was used. The exercise protocol in our centre has previously been described.12
AF-related symptoms and QoL
Patients categorised their symptoms related to AF according to the European Heart Rhythm Association (EHRA) score into 1 (no symptoms), 2a (mild symptoms), 2b (moderate symptoms), 3 (severe symptoms) and 4 (disabling symptoms). Patients completed a modified EHRA score at baseline and at monthly intervals for 12 months.
QoL was assessed by the Medical Outcomes Study Short Form-36 questionnaire (SF-36).13 Patients completed the questionnaire without assistance at baseline and monthly intervals for 12 months.
Patient procedural experience
Visual analogue scores (VAS) were used to assess procedural satisfaction and patient discomfort during and post-procedure. In patients who underwent PPM and AVNA, procedural satisfaction was assessed following AVNA and was intended to cover both procedures. The VAS comprised a 100 mm horizontal line anchored at the left with the statement ‘no discomfort’ (assigned a score of 1) and at the right with the statement ‘worst discomfort’ (assigned a score of 0). Participants were asked to look at the line and think about the discomfort they experienced during and post-procedure. There were small vertical lines along the main horizontal line at centimetre intervals and participants had to mark where they felt most accurately represented their satisfaction with the intervention.
Statistical analysis
The primary end point was recurrence of persistent AF confirmed by ICM or PPM interrogation. Persistent AF was defined as recurrence of an episode of AF lasting ≥7 days and included episodes requiring treatment to convert to normal sinus rhythm. A sample size of 75 patients was chosen to have an 80% chance of detecting a clinically significant difference in the primary outcome at one-year follow-up, defined as longest time to recurrence of persistent AF recorded by ICMs. The smallest effect size that was considered of scientific interest was a longer time to recurrence of persistent AF at three, six, nine and 12 months following intervention for AF ablation versus DCCV as recorded on ICMs. Historical data were used to inform the calculations.14,15 All efficacy analyses were based on ICM or PPM data only. Data analysis was performed using SPSS statistical software (Version 23, IBM Corp, New York, NY, USA). Baseline characteristics of all randomised subjects were summarised. Categorical variables are reported as observed number of patients (%). Continuous variables are reported as mean ± standard deviation (SD). The trial adopted a treatment policy estimand and used the intention-to-treat principle to avoid bias, as this provides an unbiased estimate of the effect of a specific treatment strategy. Categorical variables were compared using the Chi-squared test, and continuous variables compared using analysis of variance (ANOVA). Survival analyses were conducted using the Kaplan-Meier statistic and the Cox proportion hazard test and log-rank test. Post hoc logistic regression analyses were applied to explore the impact of effective restoration of SR on exercise performance. A p value <0.05 was considered significant.
Results
There were 93 patients screened (figure 1). Sixteen patients were excluded: eight did not meet the inclusion criteria and eight declined to participate. There were 77 randomised patients included in the primary efficacy analysis of patients. All patients were included in a secondary safety analysis. Baseline demographic data are displayed in table 1 and supplementary table S1. While there were no statistically significant differences between the randomised groups, there were non-significant differences in gender, duration of persistent AF and total history of AF (table 1).
Table 1. Baseline demographics for study population
| DCCV | PPM + AVNA | AF ablation | p value | |
|---|---|---|---|---|
| Number | 25 | 27 | 25 | NS |
| Mean age ± SD, years | 75.5 ± 5.5 | 76.0 ± 5.7 | 74.7 ± 5.1 | 0.710 |
| Female gender, n (%) | 7 (28) | 14 (48) | 13 (52) | 0.187 |
| Hypertension, n (%) | 19 (76) | 22 (81) | 19 (76) | 0.863 |
| Hyperlipidaemia, n (%) | 12 (48) | 11 (41) | 12 (48) | 0.836 |
| Diabetes mellitus, n (%) | 0 (0) | 2 (7) | 4 (16) | 0.110 |
| Prior cerebrovascular accident or transient ischaemic attack, n (%) | 1 (4) | 1 (4) | 2 (8) | 0.751 |
| Chronic kidney disease, n (%) | 16 (64) | 13 (48) | 14 (56) | 0.527 |
| Coronary artery disease, n (%) | 7 (28) | 7 (26) | 5 (20) | 0.799 |
| Chronic obstructive pulmonary disease, n (%) | 2 (8) | 5 (19) | 3 (12) | 0.532 |
| Mean left atrial diameter ± SD, cm | 4.22 ± 0.951 | 4.23 ± 0.813 | 4.11 ± 1.0 | 0.901 |
| Mean left atrial area ± SD, cm2 | 23.0 ± 6.151 | 26.0 ± 6.104 | 22.4 ± 6.8 | 0.158 |
| Mean LVEF pre-intervention ± SD, % | 54.3 ± 12.010 | 53.8 ± 9.561 | 50.7 ± 10.5 | 0.456 |
| Mean LVEF post-intervention ± SD, % | 57.2 ± 8.9 | 52.5 ± 9.6 | 57.0 ± 8.8 | 0.272 |
| Mean duration of persistent AF ± SD, months | 6.9 ± 5.5 | 9.1 ± 11.4 | 4.0 ± 2.3 | 0.059 |
| Mean total history of AF ± SD, months | 23.12 ± 37 | 27.2 ± 27.6 | 14.0 ± 17.9 | 0.239 |
| Key: AF = atrial fibrillation; AVNA = atrioventricular node ablation; DCCV = direct current cardioversion; LVEF = left ventricular ejection fraction; PPM = permanent pacemaker; SD = standard deviation | ||||
Supplementary table S1. Baseline medications for study population
| Medication, n (%) | DCCV | PPM | AF ablation | p value |
|---|---|---|---|---|
| BB | 15 (60) | 16 (59) | 18 (72) | 0.581 |
| ACE-i/ARB | 16 (64) | 17 (63) | 14 (56) | 0.824 |
| Calcium antagonist | 5 (20) | 6 (22) | 4 (16) | 0.854 |
| Aldosterone antagonist | 0 (0) | 2 (7) | 2 (8) | 0.371 |
| Statin | 12 (48) | 11 (41) | 12 (48) | 0.836 |
| DOAC | 18 (72) | 15 (56) | 15 (60) | 0.337 |
| Warfarin | 7 (28) | 27 (44) | 10 (40) | 0.464 |
| Key: ACE-i = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin-receptor blocker; AVNA = atrioventricular node ablation; BB = beta blockers; DCCV = direct current cardioversion; DOAC = direct oral anticoagulant; PPM = permanent pacemaker | ||||
AF burden throughout the study period
Patients in the AF ablation + amiodarone arm had a lower 12-month AF burden compared with both DCCV + amiodarone and PPM + AVNA (without amiodarone) (17.0 ± 37.9% compared with 61.7 ± 48.6% and 76.4 ± 41.6%, respectively, p<0.0001) (table 2).
Table 2. Atrial fibrillation (AF) arrhythmia outcomes
| Efficacy outcomes | DCCV N=25 |
PPM + AVNA N=27 |
p value (1 vs. 2) |
AF ablation N=25 |
p value (1 vs. 3) |
Overall p value |
|---|---|---|---|---|---|---|
| Average baseline AF burden, % | 100 | 100 | ns | 100 | ns | ns |
| Average 6-month AF burden ± SD, % |
51.1 ± 44.9 | 75.5 ± 41.2 | 0.051 | 15.9 ± 33.4 | 0.02 | 0.002 |
| Average 12-month AF burden ± SD, % |
61.7 ± 48.6 | 76.4 ± 41.6 | 0.250 | 17.0 ± 37.9 | 0.0001 | 0.0001 |
| Number of patients requiring repeat procedures in the same treatment arm, n (%) |
9 (36) | 2 (7) | 0.012 | 6 (24) | 0.359 | 0.359 |
| Number of patients switching treatment arms*, n (%) | 10 (40) | 2 (7) | 0.006 | 1 (5) | 0.002 | 0.001 |
| *See supplementary table S2 for a detailed breakdown. Key: AF = atrial fibrillation; AVNA = atrioventricular node ablation; DCCV = direct current cardioversion; PPM = permanent pacemaker; SD = standard deviation |
||||||
Time to recurrence of persistent AF
For the primary efficacy outcome of time to persistent AF recurrence, there was a statistically non-significant trend to increased efficacy of AF ablation compared with DCCV (hazard ratio [HR] 0.559, 95% confidence interval [CI] 0.293 to 1.065, p=0.073) with no difference in efficacy of DCCV compared with PPM + AVNA (HR 0.990, 95%CI 0.539 to 1.818, p=0.973) (figures 2A and 2B). Persistent AF recurred in 60%, 81.4% and 28% in the DCCV, PPM + AVNA and AF ablation arms, respectively, after the index procedure. Following redo procedures, persistent AF recurred in 16% of the AF ablation patients.


Time to recurrence of any device-derived AF
There was a trend to increased efficacy of AF ablation compared with DCCV for time to recurrence of any device-derived AF (HR 1.790, 95%CI 0.939 to 3.413, p=0.077) (figure 3).

| p value | Hazard ratio | 95%CI | |
|---|---|---|---|
| DCCV vs. PPM and AVNA | 0.9733 | 0.990 | 0.539 to 1.818 |
| DCCV vs. AF ablation | 0.0769 | 1.790 | 0.939 to 3.413 |
| Key: AF = atrial fibrillation; AVNA = atrioventricular node ablation; DCCV = direct current cardioversion; HR = hazard ratio; PPM = permanent pacemaker | |||
Procedures in each study arm
Table 2 provides details of the number of repeat procedures performed in each arm. Patients underwent more repeat procedures with DCCV compared with AF ablation and PPM + AVNA (36% for DCCV, compared with 24% for AF ablation and 7% for PPM + AVNA). Supplementary table S2 provides details about crossover rates in each of the study arms. More patients switched arms with DCCV than PPM + AVNA and AF ablation (40% for DCCV compared with 7% and 5% for PPM + AVNA and AF ablation, respectively, p=0.001).
Supplementary table S2. Crossover rates
| Randomisation group | |||
|---|---|---|---|
| Procedure subsequently performed, n (%) | DCCV | PPM + AVNA | AF ablation |
| DCCV | – | 1 (4) | 1 (5) |
| PPM + AVNA | 5 (20) | – | 0 (0) |
| AF ablation | 5 (20) | 1 (4) | – |
| Total crossover | 10 (40) | 2 (7) | 1 (5) |
| Key: AF = atrial fibrillation; AVNA = atrioventricular node ablation; DCCV = direct current cardioversion; PPM = permanent pacemaker | |||
Patient procedural experience
Procedural satisfaction was 1.0 ± 0.0 for DCCV compared with 0.3 ± 0.4 for PPM + AVNA and 0.6 ± 0.5 for AF ablation; p=0.030 and p=0.074, respectively. Post-procedural satisfaction was 1.0 ± 0.0 for DCCV and 0.4 ± 0.4 and 0.8 ± 0.5 for PPM + AVNA and AF ablation; p=0.036 and p=0.196, respectively (supplementary table S3).
CPET
No differences in baseline and six-month post-intervention VO2 peak, power reached, VE/VCO2, (minute ventilation-to-carbon dioxide output) respiratory quotient (RQ), breathing reserve (BR), maximal heart rate or VO2/HR were observed in the overall study population (table 3).
Table 3. Change in key cardiopulmonary exercise test (CPET) parameters
| Item | Estimate (vs. DCCV) | Lower 95%CI | Upper 95%CI | p value |
|---|---|---|---|---|
| PPM + AVNA | ||||
| Exercise duration, seconds | –2,711.38 | –8,700.46 | 3,277.70 | 0.37 |
| VE/VCO2 | 2.64 | –1.44 | 6.72 | 0.20 |
| Max VE, L/min | 3.74 | –2.07 | 9.56 | 0.20 |
| Power, Watts | –4.99 | –15.26 | 5.28 | 0.33 |
| VO2 max, L/min | –0.20 | –1.61 | 1.21 | 0.77 |
| RQ | 0.05 | –0.02 | 0.12 | 0.20 |
| BR, L/min | –3.66 | –9.90 | 2.58 | 0.24 |
| HR, bpm | 5.59 | –14.07 | 25.25 | 0.56 |
| VO2/HR | –0.71 | –4.80 | 3.38 | 0.72 |
| AF ablation | ||||
| Exercise duration, seconds | –2,001.58 | –8,314.30 | 4,311.14 | 0.52 |
| VE/VCO2 | –0.87 | –3.96 | 2.22 | 0.57 |
| Max VE, L/min | 3.15 | –4.14 | 10.44 | 0.39 |
| Power, Watts | –5.15 | –20.43 | 10.13 | 0.50 |
| VO2 max, L/min | 0.04 | –1.79 | 1.86 | 0.97 |
| RQ | 0.090 | 0.022 | 0.157 | 0.011 |
| BR, L/min | –4.12 | –11.46 | 3.22 | 0.26 |
| HR, bpm | 8.46 | –6.60 | 23.51 | 0.25 |
| VO2/HR | 0.21 | –2.94 | 3.37 | 0.89 |
| Key: AVNA = atrioventricular node ablation; BR = breathing reserve; CI = confidence interval; CPET = cardiopulmonary exercise test; HR = heart rate; PPM = permanent pacemaker; RQ = respiratory quotient; VCO2 = peak carbon dioxide production; VE = ventilation; VO2 peak = peak oxygen consumption | ||||
Supplementary table S3. Procedural data
| Adverse events | DCCV | PPM + AVNA | p value (1 vs. 2) | AF ablation | p value (1 vs. 3) | p value |
|---|---|---|---|---|---|---|
| Major, n | ||||||
| Death | 0 | 0 | ns | 0 | ns | ns |
| Long-term disability | 0 | 0 | ns | 0 | ns | ns |
| Total | 0 | 0 | ns | 0 | ns | ns |
| Minor, n | ||||||
| Post-operative AKI | 0 | 0 | ns | 1 | ns | ns |
| Pericarditis | 0 | 0 | ns | 0 | ns | ns |
| Infection | 0 | 0 | ns | 0 | ns | ns |
| Femoral vascular complication | 0 | 0 | ns | 1 | ns | ns |
| Mean procedural satisfaction ± SD | 1 ± 0.0 | 0.3 ± 0.4 | 0.030 | 0.6 ± 0.5 | 0.074 | 0.045 |
| Mean post-procedural satisfaction ± SD | 1 ± 0.0 | 0.4 ± 0.4 | 0.036 | 0.8 ± 0.5 | 0.196 | 0.137 |
| Key: AF = atrial fibrillation; AKI = acute kidney injury; AVNA = atrioventricular node ablation; DCCV = direct current cardioversion; PPM = permanent pacemaker; SD = standard deviation | ||||||
The effect of restoration of sinus rhythm on CPET parameters (post-hoc analyses)
Twenty-six patients (nine DCCV, two PPM + AVNA and 15 AF ablation) were in AF at baseline CPET and subsequently SR at six-month CPET confirmed on ICM. SR at six months was an independent predictor for a lower VE/VCO2 (39.7 ± 9.9 to 33.3 ± 5.0 ml/kg/min, p=0.004), an increased power (64.4 ± 37.4 to 89.6 ± 37.3 Watts, p=0.014) and a higher VO2 peak (12.1 ± 4.2 to 15.3 ± 4.2 ml/kg/min, p=0.013) (table 4).
Table 4. The impact of sinus rhythm on exercise performance
| Mean ± SD | AF at 6 months | SR at 6 months | Adjusted R squared | df | 95%CI lower bound | 95%CI upper bound | p value |
|---|---|---|---|---|---|---|---|
| VE/VCO2 | 39.7 ± 9.9 | 33.3 ± 5.0 | 0.129 | 54 | –10.769 | –2.141 | 0.004 |
| max VE, L/min | 43.1 ± 16.2 | 50.0 ± 16.0 | 0.027 | 56 | –1.762 | 15.427 | 0.117 |
| Power, Watts | 64.4 ± 37.4 | 89.6 ± 37.3 | 0.089 | 56 | 5.323 | 45.121 | 0.014 |
| VO2 peak, L/min | 12.1 ± 4.2 | 15.3 ± 4.2 | 0.111 | 46 | 0.709 | 5.632 | 0.013 |
| BR, L/min | 50.8 ± 13.0 | 46.4 ± 11.7 | 0.013 | 54 | –11.1 | 2.315 | 0.194 |
| HR, bpm | 96.5 ± 23.2 | 103.5 ± 14.2 | 0.008 | 45 | –4.983 | 18.806 | 0.248 |
| VO2/HR | 10.5 ± 3.7 | 12.3 ± 3.6 | 0.033 | 38 | –0.614 | 4.202 | 0.140 |
| Key: AF = atrial fibrillation; BR = breathing reserve; CI = confidence interval; Df = degree of freedom; RQ = respiratory quotient; HR = heart rate; SD = standard deviation; SR = sinus rhythm; VCO2 = peak carbon dioxide production; VE = ventilation; VO2 peak = peak oxygen consumption | |||||||
Symptom questionnaires
There was an improvement in EHRA class across all arms of the study (2.6 ± 0.5 at baseline compared with 2.0 ± 0.6 at 12 months for DCCV, p=0.008; 2.44 ± 0.5 to 1.9 ± 0.5 for PPM + AVNA, p=0.004, and 2.3 ± 0.4 to 1.7 ± 0.6 for AF ablation, p=0.006). For the overall study population, baseline mean modified EHRA score was 2.4 ± 0.495 and at 12 months follow-up was 1.84 ± 0.081 (p=0.00001).
Following AF ablation, eight patients had a final AF burden of >1%. In these patients, the EHRA class improved from 2.7 ± 0.27 to 2.1 ± 0.074 (p=0.037). Following DCCV, 11 patients had a final AF burden of >1%. The EHRA class improved from 2.69 ± 0.40 to 2.25 ± 0.66 (p=0.0011).
Safety
One patient randomised to DCCV died two months following their intervention. The cause of death was sepsis secondary to diabetic foot ulceration.
There were no significant differences in procedural safety analysis (supplementary table S3). In the AF ablation arm, one patient developed an acute kidney injury post-operatively managed conservatively, and another developed a right femoral pseudoaneurysm requiring thrombin injection. In the PPM + AVNA arm, one patient suffered an atrial lead displacement and in another the AV node recovered. These patients underwent redo procedures.
During the study period, the numbers of patients who developed an intolerance to amiodarone in the DCCV and ablation arms were eight (32%) and 10 (40%), respectively.
Discussion
This randomised-controlled trial compared DCCV versus AF ablation versus AVNA + PPM in patients ≥65 years with persistent AF utilising continuous monitoring with ICMs. The major findings of the study are:
- A significant reduction in AF burden following cryoablation compared with DCCV.
- A non-significant trend to reduced recurrence of persistent AF following AF cryoablation compared with DCCV.
- A non-significant trend to reduced recurrence of ≥2 minutes device-derived AF following AF cryoablation compared with DCCV.
- An improvement in functional status and QoL across all treatment groups.
- Restoration of SR was an independent predictor of improvement in key prognostic facets of exercise performance at six-month CPET.
Efficacy of catheter ablation
AF ablation utilising cryotherapy with concomitant amiodarone therapy resulted in good AF outcomes, with successful abolition of persistent AF in 72% of patients at 12-month follow-up using a stringent threshold set. Most patients not in SR at 12 months, were in paroxysmal AF, albeit with a low mean burden. Within a year, 24% (6/25) underwent repeat ablation, and this is consistent with other studies.16 With repeat ablations, the success rate reached 84%. Our data further suggest the value of left atrial cryoablation as an effective and safe procedure in elderly patients with persistent AF.17,18
Most patients (17/25) had a final AF burden of <1%. Measurement is only adequately achieved with implanted devices capable of continuous heart rhythm monitoring. While our study demonstrates a reduction in AF burden, further studies are required to ascertain whether this translates to a reduction in hard clinical outcomes, such as mortality.
DCCV vs. AF ablation
While statistically non-significant, our data indicated a trend to reduced recurrence of AF following ablation compared with DCCV. Patients treated with AF ablation were less likely to switch treatment arms and required fewer redo procedures than those with DCCV. These findings may suggest a benefit of AF ablation over DCCV in the study’s population.
There was a significant reduction in AF burden at 12 months with cryoablation compared with DCCV. Most studies evaluate AF in a binary fashion (present or absent) and do not have the ability to investigate AF burden utilising ICMs, as in the current trial. This finding is important because a higher AF burden is associated with an increased risk of stroke, heart failure and mortality.19 While further study is required, this suggests a potential benefit of cryoablation over DCCV in patients with persistent AF ≥65 years of age because the final AF burden was lower in this treatment arm. There were no differences in symptom scores between the groups suggesting that AF burden may not correlate with QoL. These findings differ to a CAPTAF (Catheter Ablation compared with Pharmacological Therapy for Atrial Fibrillation) trial substudy, which found a significant association between AF burden and QoL.20 The precise relationship between AF burden and QoL remains to be elucidated, presenting an exciting area for further study.
Cryoablation was associated with a 24% redo ablation rate in the current study, which is in keeping with other studies assessing cryoablation in persistent AF.21 In the future, studies should determine the subgroups of the persistent AF population that are likely to require redo ablation procedures. A personalised approach to patient management may result in higher success rates.
DCCV vs. PPM + AVNA
DCCV was common to both treatment arms allowing a fair comparison of the true efficacy of each intervention. There were no significant differences in AF burden between DCCV and PPM + AVNA. Our study suggests that it is reasonable to employ either modality in persistent AF patients, particularly in instances where catheter ablation may not be suitable. Previous studies have shown that oral amiodarone can increase the efficacy of electrical cardioversion.22 It is worth noting that almost a third (8/25) of the DCCV patients developed an intolerance to amiodarone, which may have contributed to the results obtained. This finding asserts the external validity of our study, as amiodarone is often poorly tolerated. In situations where the use of long-term amiodarone may be unsuitable, our study demonstrates that PPM and AVNA may be a feasible alternative, obviating the requirement for long-term anti-arrhythmic drug use.
Global haemodynamic and functional improvement
SR was an independent predictor for a higher VO2 peak at six-month CPET. This aspect of exercise performance has been shown to have high prognostic value in cardiac patients and healthy individuals,23 and this finding is in keeping with recent publications.24 The results imply that a SR-associated haemodynamic improvement may improve the rate of O2 transport.
SR was an independent predictor for a significant reduction in the VE/VCO2 slope at six-month CPET. This important index of ventilator response to exercise is a risk predictor for congestive heart failure, a condition commonly associated with AF.23 An increase in VE/VCO2 in AF is at least partly explained by a decrease in pulmonary perfusion.25 The improvement in cardiovascular haemodynamics and pulmonary perfusion with SR explains the lower VE/VCO2 observed and this provides a potential explanation for the prognostic benefit of AF ablation in patients with AF and heart failure observed in the CASTLE-AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial.25 Future studies should attempt to determine whether the lower VE/VCO2 observed in the current study translates to improvements in morbidity and mortality.
The power reached on the cycle ergometer increased with restoration of SR. This finding highlights the value of SR in improving cardiovascular efficiency, resulting in a comprehensive improvement in exercise tolerance.26 Improvements in exercise tolerance may enable AF patients to partake in more exercise. The importance of this has been affirmed by the ACTIVE-AF (A Lifestyle-based, PhysiCal AcTIVity IntErvention for Patients With Symptomatic Atrial Fibrillation) trial,27 which demonstrated significantly lower AF symptom severity in patients who participated in a supervised exercise programme. Our observed improvement in exercise tolerance may, therefore, provide an explanation for the improvement in QoL in patients achieving successful SR restoration.28
Quality of life
Prior studies using the SF-36 for predominantly paroxysmal AF have shown a significant post-ablation amelioration of QoL, with the benefit lasting over two years.29 QoL improved significantly in all treatment arms with no differences between groups. Previous studies have shown that QoL improves over time irrespective of rate or rhythm control, though this may relate to the low sensitivity of the generic instruments to symptom changes in AF patients.8
There was an improvement in modified EHRA score across all treatment groups, but no difference between groups. Previous studies have demonstrated that symptom improvement can occur with little or no effect on AF burden.30 The improvement in symptomatic status could, therefore, be related to successful rhythm control or robust rate control in the PPM + AVNA arm.
Study limitations
One limitation of our study appears to have been the sample size. While historical data were used to inform the power calculations and sample size, it appears that the trial may indeed have been underpowered to exclude significant differences both in baseline variables and outcomes. Upon examination of the 95% confidence intervals around the point estimates of the primary outcome, we acknowledge that the values obtained could lead to different inferential conclusions. Nevertheless, we believe that the results are of interest and should be examined in further, large-scale studies.
A further limitation of the study was that it comprised three heterogeneous treatment arms. This meant that the study was unblinded, and this may have confounded the results. Patients in the PPM + AVNA arm did not receive amiodarone therapy, which may have affected the results. Furthermore, patients in this arm appeared to have relatively high rates of AF recurrence. There was also a high rate of crossover to different treatment arms during the study. Despite the use of an intention-to-treat analysis, the crossover rate may have affected the results obtained.
Our study did not include a rate-control therapy arm, as it would be natural to surmise that such a modality would be unable to achieve the primary end point. The use of medications for rate control was not statistically significant in the study (supplementary table S1). Further studies should investigate the impact of rate-control therapy on some of the outcomes measured in this trial in persistent AF patients aged ≥65 years.
There was a discrepancy in monitoring techniques with ICMs utilised in the DCCV and cryoablation arms and an atrial PPM lead in the PPM + AVNA arm. PPMs have previously been shown to have superior AF detection,31 which could potentially bias results. Furthermore, Medtronic Reveal XT ICMs are not capable of detecting isolated episodes of AF lasting <2 minutes unless a manual activation of the device is made by the patient. Recent evidence has demonstrated that the presence of short-lasting episodes of AF-like activity lasting <30 seconds indicates increased likelihood for undetected AF and, therefore, could be of clinical relevance.32 These short-lived episodes could have been missed on the ICMs.
Conclusion
Cryoablation was associated with a lower AF burden than DCCV at 12 months. A non-significant trend towards reduced AF recurrence was observed with AF ablation compared with DCCV. While further study is required to ascertain the impact of these findings on outcomes such as stroke and/or mortality, these data suggest that cryoablation may be useful in persistent AF patients aged ≥65 years. DCCV was better tolerated by patients, but was associated with more chance of patients requiring repeat treatment and alternative therapies. SR restoration was associated with a significant increase in VO2 peak and power, and a significant reduction in VE/VCO2, suggesting improved ventilatory response to exercise in SR, providing an interesting hypothesis for the prognostic benefit of catheter ablation in patients with AF and heart failure.
Key messages
- In comparison to direct current cardioversion (DCCV), cryoablation was associated with a significant reduction in atrial fibrillation (AF) burden in persistent AF patients ≥65 years
- A non-significant trend towards reduced AF recurrence was observed in patients treated with AF cryoablation compared with DCCV
- While further studies are required, our data suggest cryoablation may be a beneficial treatment approach in persistent AF patients ≥65 years
- Irrespective of treatment strategy, sinus rhythm restoration was an independent predictor of improvement in key prognostic facets of exercise performance at six-month cardiopulmonary exercise test (CPET)
Conflicts of interest
None declared.
Funding
We acknowledge the support of the Eastbourne Cardiology Research Charity Fund (ECRCF) which provided an unrestricted grant to fund the study.
Study approval
The study protocol was approved by the institutional review board and national ethics committee (clinicaltrials.gov identifier NCT02528604, REC reference 16/LO/0727, IRAS project ID 195936). All patients gave written informed consent.
References
1. Amin A, Houmsse A, Ishola A, Tyler J, Houmsse M. The current approach of atrial fibrillation management. Avicenna J Med 2016;6:8–16. https://doi.org/10.4103/2231-0770.173580
2. Nault I, Miyazaki S, Forclaz A et al. Drugs vs. ablation for the treatment of atrial fibrillation: the evidence supporting catheter ablation. Eur Heart J 2010;31:1046–54. https://doi.org/10.1093/eurheartj/ehq079
3. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612
4. Brandes A, Crijns HJGM, Rienstra M et al. Cardioversion of atrial fibrillation and atrial flutter revisited: current evidence and practical guidance for a common procedure. Europace 2020;22:1149–61. https://doi.org/10.1093/europace/euaa057
5. Channer KS, Birchall A, Steeds RP et al. A randomized placebo-controlled trial of pre-treatment and short- or long-term maintenance therapy with amiodarone supporting DC cardioversion for persistent atrial fibrillation. Eur Heart J 2004;25:144–50. https://doi.org/10.1016/j.ehj.2003.10.020
6. Ozcan C, Jahangir A, Friedman PA et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med 2001;344:1043–51. https://doi.org/10.1056/NEJM200104053441403
7. Vlachos K, Letsas KP, Korantzopoulos P, Liu T, Efremidis M, Sideris A. A review on atrioventricular junction ablation and pacing for heart rate control of atrial fibrillation. J Geriatr Cardiol 2015;12:547–54. https://doi.org/10.11909/j.issn.1671-5411.2015.05.005
8. Camm AJ, Kirchhof P, Lip GYH et al. Guidelines for the management of atrial fibrillation: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. https://doi.org/10.1093/europace/euq350
9. Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017;33:369–409. https://doi.org/10.1016/j.joa.2017.08.001
10. Boodhoo L, Mitchell ARJ, Bordoli G, Lloyd G, Patel N, Sulke N. DC cardioversion of persistent atrial fibrillation: a comparison of two protocols. Int J Cardiol 2007;114:16–21. https://doi.org/10.1016/j.ijcard.2005.11.108
11. Terricabras M, Verma A, Morillo CA. Measuring success in ablation of atrial fibrillation: time for a paradigm shift? Circ Arrhythm Electrophysiol 2018;11:e006582. https://doi.org/10.1161/CIRCEP.118.006582
12. Eysenck W, van Zalen J, Freemantle N, Lloyd G, Furniss S, Sulke N. The hemodynamic effects of a central iliac arteriovenous anastomosis at 6 months in patients with resistant and uncontrolled hypertension. J Clin Hypertens (Greenwich) 2019;21:1399–405. https://doi.org/10.1111/jch.13646
13. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med 2016;4:2050312116671725. https://doi.org/10.1177/2050312116671725
14. Veasey RA, Silberbauer J, Schilling RJ et al. The evaluation of pulmonary vein isolation and wide-area left atrial ablation to treat atrial fibrillation in patients with implanted permanent pacemakers: the Previously Paced Pulmonary Vein Isolation study. Heart 2010;96:1037–42. https://doi.org/10.1136/hrt.2009.188425
15. Verma A, Minor S, Kilicaslan F et al. Incidence of atrial arrhythmias detected by permanent pacemakers (PPM) post-pulmonary vein antrum isolation (PVAI) for atrial fibrillation (AF): correlation with symptomatic recurrence. J Cardiovasc Electrophysiol 2007;18:601–06. https://doi.org/10.1111/j.1540-8167.2007.00789.x
16. Abdin A, Yalin K, Lyan E et al. Safety and efficacy of cryoballoon ablation for the treatment of atrial fibrillation in elderly patients. Clin Res Cardiol 2019;108:167–74. https://doi.org/10.1007/s00392-018-1336-x
17. Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J 2017;38:20–6. https://doi.org/10.1093/eurheartj/ehw260
18. Wynn GJ, El-Kadri M, Haq I et al. Long-term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Heart 2016;3:e000394. https://doi.org/10.1136/openhrt-2015-000394
19. Boriani G, Diemberger I, Ziacchi M et al. AF burden is important – fact or fiction? Int J Clin Pract 2014;68:444–52. https://doi.org/10.1111/ijcp.12326
20. Jansson V, Bergfeldt L, Schwieler J et al. Atrial fibrillation burden, episode duration and frequency in relation to quality of life in patients with implantable cardiac monitor. Int J Cardiol Heart Vasc 2021;34:100791. https://doi.org/10.1016/j.ijcha.2021.100791
21. Sawhney V, Schilling RJ, Providencia R et al. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace 2020;22:375–81. https://doi.org/10.1093/europace/euz313
22. Capucci A, Villani GQ, Aschieri D, Rosi A, Piepoli MF. Oral amiodarone increases the efficacy of direct-current cardioversion in restoration of sinus rhythm in patients with chronic atrial fibrillation. Eur Heart J 2000;21:66–73. https://doi.org/10.1053/euhj.1999.1734
23. Sarullo FM, Fazio G, Brusca I et al. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J 2010;4:127–34. https://doi.org/10.2174/1874192401004010127
24. Fiala M, Wichterle D, Bulková V et al. A prospective evaluation of haemodynamics, functional status, and quality of life after radiofrequency catheter ablation of long-standing persistent atrial fibrillation. Europace 2014;16:15–25. https://doi.org/10.1093/europace/eut161
25. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;379:492. https://doi.org/10.1056/NEJMc1806519
26. Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol 2017;70:1618–36. https://doi.org/10.1016/j.jacc.2017.08.012
27. Elliott A, Verdicchio C, Mahajan R et al. An exercise and physical activity program in patients with atrial fibrillation: the ACTIVE-AF randomized controlled trial. J Am Coll Cardiol EP 2023;9:455–65. https://doi.org/10.1016/j.jacep.2022.12.002
28. Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C. Exercise and heart failure: an update. ESC Heart Fail 2018;5:222–32. https://doi.org/10.1002/ehf2.12225
29. Weerasooriya R, Jaïs P, Hocini M et al. Effect of catheter ablation on quality of life of patients with paroxysmal atrial fibrillation. Heart Rhythm 2005;2:619–23. https://doi.org/10.1016/j.hrthm.2005.02.1037
30. Björkenheim A, Brandes A, Magnuson A et al. Assessment of atrial fibrillation-specific symptoms before and 2 years after atrial fibrillation ablation: do patients and physicians differ in their perception of symptom relief? JACC Clin Electrophysiol 2017;3:1168–76. https://doi.org/10.1016/j.jacep.2017.04.003
31. Podd SJ, Sugihara C, Furniss SS, Sulke N. Are implantable cardiac monitors the “gold standard” for atrial fibrillation detection? A prospective randomized trial comparing atrial fibrillation monitoring using implantable cardiac monitors and DDDRP permanent pacemakers in post atrial fibrillation. Europace 2016;18:1000–05. https://doi.org/10.1093/europace/euv367
32. Fredriksson T, Gudmundsdottir KK, Frykman V et al. Brief episodes of rapid irregular atrial activity (micro-AF) are a risk marker for atrial fibrillation: a prospective cohort study. BMC Cardiovasc Disord 2020;20:167. https://doi.org/10.1186/s12872-020-01453-w
