Factor (F) XI or XIa inhibition has attracted interest due to the protection from thrombotic events and minimal bleeding tendency observed in FXI-deficient individuals. The prospect of uncoupling the management of thrombosis from the bleeding risk inadvertently associated with current therapy inspired the development of agents directed towards this step in the coagulation process. This review describes the physiological rationale behind FXI/FXIa inhibition and the pharmacological properties of existing FXI/FXIa inhibitors. It also explores the potential clinical use of these agents in various thromboembolic pathologies, predominantly through the phase II clinical trials conducted so far comparing them to current anticoagulant therapy or placebo.
Introduction

Anticoagulant therapy is an essential component in the treatment and prevention of venous and arterial thromboembolic events. In recent years, direct-acting oral anticoagulants (DOACs) have replaced vitamin K antagonists (VKAs) for many of these indications, due to their more favourable risk-benefit profile.1 Despite this, bleeding remains a significant concern with DOACs, especially in patients at high risk, such as those with an indication for concurrent antiplatelet therapy, and may lead to poor adherence or undertreatment.2–4 Safer anticoagulation that spares haemostasis without compromising efficacy is, therefore, desirable. The zymogen factor (F) XI and its activated serine protease form (FXIa) have emerged as targets for potential therapies to address this issue.
Factor XI as a target for anticoagulation therapy
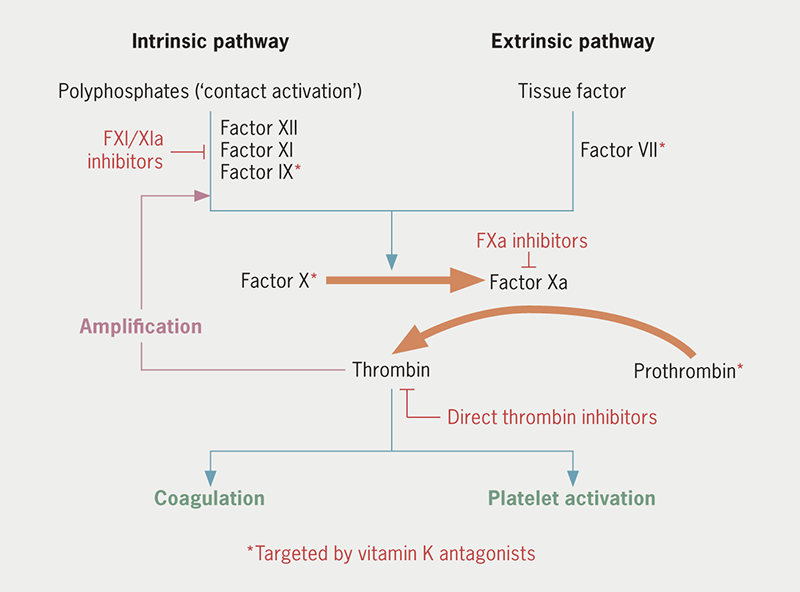
FXI is mainly synthesised in hepatocytes and can be activated both by activated factor XII (FXIIa), as part of the intrinsic pathway of coagulation, and by thrombin itself in a positive feedback loop (figure 1).5 FXIa then activates FIX, sustaining thrombin generation.6,7 FXI has a different level of contribution to the pathways of haemostasis and thrombosis. In haemostasis, there is binding of FVII or FVIIa to extravascular tissue factor (TF) at the site of injury causing thrombin generation via the extrinsic pathway of coagulation. The FVIIa-TF complex also activates FIX, which sustains the thrombus through further FX activation. The feedback activation of FXI by thrombin is of little significance to this process (and the role of FXIIa even less so). In contrast, pathological thrombosis is associated with triggers within vessel walls or cardiac chambers. Low-shear settings, such as venous or left atrial thrombosis, may involve stasis, hypoxia and endothelial damage, all leading to the accumulation or upregulation of procoagulants, including TF, while arterial thrombosis is usually the result of atherosclerotic plaque disruption.8,9 Exposure to TF in these intraluminal environments leads to the generation of thrombin that is far more dependent on feedback activation of FXI to sustain it, possibly because the thrombus propagates inwards and away from the initial TF source. This process is further enhanced by polyphosphates released from activated platelets and chromatin released from activated neutrophils activating FXII, in turn activating FXI.10,11 In patients with haemophilia C or congenital FXI deficiency, reduced FXI activity (≤50%) was associated with lower rates of cardiovascular events (adjusted hazard ratio 0.57 for ≤30% activity; 0.52 for 30–50% activity) and lower rates of venous thromboembolism (VTE) (adjusted hazard ratio 0.26 for ≤50% activity). A history of gastrointestinal bleeding was more likely with FXI deficiency, but rates of intracerebral haemorrhage were comparable with people with normal FXI activity.12 Bleeding is also more likely related to trauma and rarely spontaneous.12,13
Factor XI and XIa inhibitors
FXI can be targeted by reducing FXI biosynthesis or directly inhibiting FXI/FXIa (figure 2). Novel agents have been developed with different pharmacodynamic and pharmacokinetic properties (table 1). IONIS-FXIRX or FXI-ASO is a second-generation antisense oligonucleotide that binds to FXI messenger RNA, causing its degradation and thus reducing hepatic FXI synthesis.14 It is administered subcutaneously and takes approximately three to four weeks for plasma FXI activity to be reduced by 75–80% in non-human primates.15
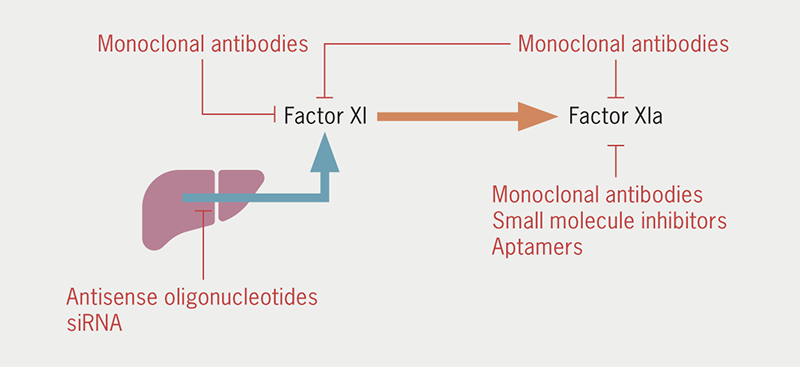
Table 1. Pharmacokinetic and pharmacodynamic properties of factor (F) XI and FXIa inhibitors
| Drug (class)/ manufacturer |
Mechanism of action | Route of administration | Onset of action | Half-life | Renal excretion | CYP metabolism | Drug interactions | Refs |
|---|---|---|---|---|---|---|---|---|
| IONIS-FXIRX (ASO)/ Ionis Pharmaceuticals |
Prevents biosynthesis of FXI | SC | Slow (weeks) | Long (weeks) | No | No | Not known | 14, 15, 26 |
| Osocimab (monoclonal antibody)/ Bayer |
Binds to FXIa near active site preventing activation of FIX | IV or SC | Rapid (hours) | Long (weeks) | No | No | Not known | 17 |
| Abelacimab (monoclonal antibody)/ Anthos Therapeutics |
Binds to FXI preventing activation by FXIIa or thrombin | IV or SC | Rapid (hours) | Long (weeks) | No | No | Not known | 18, 19 |
| Milvexian (small molecule)/ Bristol Myers Squibb & Janssen |
Direct active-site inhibitor of FXIa | Oral | Rapid (hours) | Short (hours) | Some (<20%) | CYP3A4 substrate | CYP3A inducers and inhibitors | 22, 23, 45, 46 |
| Asundexian (small molecule)/ Bayer |
Direct active-site inhibitor of FXIa | Oral | Rapid (hours) | Short (hours) | Some (<15%) | In vitro weak-to-moderate CYP3A4 inducer | Possible but unlikely | 25 |
| Key: ASO = antisense oligonucleotide; CYP = cytochrome P450; F = factor; IV = intravenous; SC = subcutaneous | ||||||||
Monoclonal antibodies, such as osocimab, abelacimab and xisomab, when administered intravenously, have a much faster onset of action with sustained FXI inhibition for several weeks. Osocimab binds to a region adjacent to the active site of FXIa, thus, preventing its activation of FIX.16 It reaches maximum plasma concentration within one to four hours after intravenous infusion and has a half-life of 30–44 days.17 Conversely, abelacimab binds to the catalytic domain of FXI, inhibiting its activation by FXIIa or thrombin. The maximum plasma concentration of abelacimab is achieved at 1.75 to two hours after commencing intravenous administration and its half-life is approximately 20–30 days.18,19 Xisomab has a somewhat different mode of action, inhibiting FXIIa-mediated, but not thrombin-mediated, FXI activation.20 It has only been assessed in a small phase II randomised, double-blind trial involving patients with end-stage renal disease (ESRD) undergoing heparin-free haemodialysis, and was associated with less frequent occlusive thrombotic events requiring circuit exchange compared with placebo.21
Milvexian and asundexian are oral small-molecule FXIa inhibitors that reversibly inhibit FXIa. Milvexian is a selective direct inhibitor of FXIa that achieves a maximum plasma concentration at two to four hours and has a half-life of 12–15 hours. Milvexian is well-tolerated, including in patients with moderate hepatic impairment.22,23 Asundexian has a similar mode of action, a half-life of 14–18 hours and achieves maximum inhibition of FXIa activity after 1.5 to four hours.24,25
A small interfering RNA (siRNA)-based agent targeting FXI (RBD4059) is planned for a phase I trial (NCT05653037) and this may eventually lead to the availability of therapies requiring much less frequent administration.
Phase II trials
Most of these agents have now undergone phase II clinical trials for various potential indications (table 2).
Table 2. Phase II clinical trials of FXI/FXIa inhibitors
| Trial name | No. of patients | FXI/FXIa inhibitor | Comparator | Efficacy outcome results | Safety outcome results |
|---|---|---|---|---|---|
| Venous thromboembolism prophylaxis | |||||
| FXI-ASO TKA | 300 (293 received trial medication) | IONIS-FXIRX | Enoxaparin SC 40 mg | Noninferiority of IONIS-FXIRX 200 mg; superiority of 300 mg IONIS-FXIRX | Similar bleeding rates |
| FOXTROT | 813 (787 received trial medication) | Osocimab IV | Enoxaparin SC 40 mg OD or apixaban orally 2.5 mg BID | Noninferiority of post-operative 0.6, 1.2 or 1.8 mg/kg osocimab; superiority of pre-operative 1.8 mg/kg osocimab | Lower bleeding rates for osocimab, rising at higher doses |
| ANT-005 TKA | 412 (409 received trial medication) | Abelacimab IV | Enoxaparin SC 40 mg | Noninferiority of 30 mg abelacimab; superiority of 75 mg and 150 mg abelacimab | Similar bleeding rates |
| AXIOMATIC-TKR | 1,242 (1,219 received trial medication) | Milvexian | Enoxaparin SC 40 mg | Superiority of milvexian total daily dose 100 mg or higher | Similar bleeding rates |
| Atrial fibrillation | |||||
| ANT-004 | 18 | Abelacimab SC | Placebo | NA | No clinically relevant bleeding events |
| PACIFIC-AF | 755 (753 received trial medication) | Asundexian | Apixaban 5 mg BID | NA | Lower bleeding rates with asundexian (pooled) |
| Acute myocardial infarction | |||||
| PACIFIC-AMI | 1,601 (1,593 received trial medication) | Asundexian | Placebo | No difference in composite of cardiovascular death, MI, stroke or stent thrombosis | Similar bleeding rates |
| Ischaemic stroke | |||||
| PACIFIC-STROKE | 1,808 (1,786 received trial medication) | Asundexian | Placebo | No difference in composite of incident MRI-detected covert brain infarcts or recurrent symptomatic ischaemic stroke | Similar bleeding rates |
| AXIOMATIC-SSP | 2,366 | Milvexian | Placebo | Awaiting published results | Awaiting published results |
| Key: BID = twice daily; IV = intravenous; MI = myocardial infarction; MRI = magnetic resonance imaging; OD = once daily; SC = subcutaneous | |||||
FXI inhibitors for VTE prophylaxis and treatment
Earlier FXI inhibitor phase II trials centred around VTE prophylaxis in patients undergoing total knee replacement (TKR) due to the relatively high number of such events post-operatively and ease of evaluation with venography. The first trial, FXI-ASO TKA, compared IONIS-FXIRX 200 or 300 mg with enoxaparin 40 mg in 300 patients undergoing unilateral TKR in an open-label design.14 Patients randomised to IONIS-FXIRX received subcutaneous doses starting 35 days before the surgery: three doses in the first week followed by once-weekly doses in the lead-up to surgery, a dose six-hours post-operatively and a final dose three days later. The incidence of VTE, assessed by venography 8–12 days post-operatively, was similar in the 200 mg IONIS-FXIRX and enoxaparin groups (27% vs. 30%, p=0.59) but significantly lower in the 300 mg IONIS-FXIRX group (4%, p<0.001). Clinically relevant bleeding was not significantly different between the three groups. Injection site reactions with IONIS-FXIRX were reportedly frequent but mild and inconsequential. A second phase II trial with IONIS-FXIRX in 49 ESRD patients demonstrated similar pharmacokinetics before and after haemodialysis with no drug accumulation or treatment-related serious adverse events (SAEs).26 The ligand-conjugated version of IONIS-FXIRX, known as fesomersen, is also undergoing studies in ESRD (NCT04534114).
The phase II FOXTROT (FactOr XIa inhibiTion for the pRevention of venOus Thromboembolism in patients undergoing total knee arthroplasty) randomised-controlled trial had an open-label, parallel-group, adaptive design and aimed to determine noninferiority of osocimab to enoxaparin for VTE prophylaxis after TKR.27 There were 813 patients randomised to receive one of the following regimens: a single pre-operative intravenous dose of osocimab at 0.3 or 1.8 mg/kg; a single post-operative intravenous dose of osocimab at 0.3, 0.6, 1.2 or 1.8 mg/kg; enoxaparin 40 mg subcutaneously daily until venography; or apixaban 2.5 mg orally twice daily until venography. The three higher post-operative doses of osocimab demonstrated non-inferiority while the higher pre-operative dose demonstrated superiority to enoxaparin regarding the primary outcome of post-operative incidence of VTE at 10–13 days. Clinically relevant bleeding for the various doses of osocimab occurred in up to 4.7% of patients compared with 5.9% receiving enoxaparin and 2% receiving apixaban. SAEs had a similar distribution across the groups. Another phase II trial to determine safety and pharmacodynamics of osocimab in patients with ESRD receiving haemodialysis has recently been completed (NCT04523220).
ANT-005 TKA was a phase II randomised, open-label, parallel-group trial comparing three doses of abelacimab, 30, 75 or 150 mg, with enoxaparin 40 mg in patients undergoing unilateral TKR.28 There were 412 patients who underwent randomisation in a 1:1:1:1 ratio to receive one of the three doses of abelacimab as a single intravenous infusion administered four to eight hours after surgery or once-daily subcutaneous enoxaparin at 40 mg administered subcutaneously until venography performed 10–12 days after surgery. Abelacimab met criteria for noninferiority to enoxaparin for all three dosage regimens while the higher two doses met criteria for superiority. The rate of VTE was 13, 5 and 4% in the groups receiving abelacimab at 30, 75 and 150 mg, respectively, compared with 22% in the enoxaparin group. Clinically relevant bleeding events were experienced by 2% of patients in the 30 and 75 mg abelacimab groups, while none occurred in the enoxaparin and 150 mg abelacimab groups. There were five treatment-emergent SAEs in the abelacimab groups. Two phase III trials are currently investigating treatment of cancer-associated VTE with abelacimab compared with apixaban (NCT05171049) and dalteparin (NCT05171075).
The largest phase II trial related to VTE prophylaxis so far was AXIOMATIC-TKR, which investigated milvexian at seven different dosing regimens. The trial randomised 1,242 patients undergoing TKR to either milvexian 25, 50 or 200 mg once daily or milvexian 25, 50, 100 or 200 mg twice daily, compared with subcutaneous enoxaparin 40 mg once daily.29 The trial had a randomised, parallel-group, adaptive design and was open-label for treatment assignment but patients and observers were blinded to the milvexian dose regimen. Patients received trial medication 12–24 hours after surgery and continued treatment for 10–14 days. There was a significant dose-dependent reduction in the incidence of VTE with both once- and twice-daily dosing regimens of milvexian. The twice-daily regimen of milvexian was superior to enoxaparin at reducing rates of post-operative VTE (12% vs. 21%), which were significantly lower than the prespecified benchmark of 30% (both one-sided p<0.001). VTE developed in 16% of total patients in the once-daily milvexian groups. Clinically relevant bleeding was reported in 1% of patients receiving milvexian (none being major) and 2% of patients receiving enoxaparin. SAEs were reported in 2% of patients on milvexian.
Owing to their similar study populations, these four phase II trials were included in an exploratory meta-analysis assessing the efficacy and safety of pooled FXI/FXIa inhibitors for VTE prophylaxis compared with enoxaparin.30 The primary efficacy end point of VTE was reduced with a FXI/FXIa inhibitor compared with enoxaparin (risk ratio [RR] 0.59, 95% confidence interval [CI] 0.37 to 0.94, p=0.038) as was the primary safety end point of clinically relevant bleeding (RR 0.41, 95%CI 0.19 to 0.92, p=0.039). Another meta-analysis similarly reported superior efficacy of FXI/FXIa inhibitors compared with enoxaparin (RR 0.62, 95%CI 0.49 to 0.79) mostly driven by the higher doses.31 Bleeding events were reduced with FXI/FXIa inhibitors (RR 0.49, 95%CI 0.31 to 0.77), but there was no difference compared with enoxaparin when only major bleeding was considered (RR 0.96, 95%CI 0.41 to 2.28).
FXI/FXIa inhibition for atrial fibrillation
Atrial fibrillation (AF) is a common indication for anticoagulation due to the increased risk of cardioembolic stroke secondary to the formation of atrial thrombi. Current guidelines recommend the use of DOACs in eligible patients in preference to VKAs in non-valvular AF.32-34 A small phase II trial of once-monthly subcutaneous abelacimab versus placebo for three months in patients with AF, demonstrated safety of the 120 and 180 mg doses with no reported major or clinically relevant non-major bleeding events.19
PACIFIC-AF is the largest phase II trial to investigate the use of FXI inhibitors in patients with AF so far. The trial had a randomised, double-blind, double-dummy design and compared two once-daily dosing regimens of the direct FXIa inhibitor asundexian (20 and 50 mg) with the direct factor Xa inhibitor apixaban at standard dosing in a 1:1:1 ratio.35 A total of 753 patients received study medication for 12 weeks. There were no major bleeding events according to the International Society on Thrombosis and Haemostasis (ISTH) criteria and 10 clinically relevant non-major bleeding events. Ratios of incidence proportions for the primary end point, a composite of ISTH major and clinically relevant non-major bleeding, were 0.5 for asundexian 20 mg, 0.16 for asundexian 50 mg and 0.33 for pooled asundexian doses, compared with apixaban. Similar results favouring asundexian were obtained when all bleeding events, including minor, were analysed. Rates of adverse events, including those leading to study drug discontinuation, were similar in all three groups. The trial was not powered to test thrombotic end points.
Another phase II trial in patients with AF is currently investigating the safety of abelacimab compared with rivaroxaban (NCT04755283).
FXI inhibitors for acute coronary syndromes
Standard treatment following acute coronary syndromes (ACS) is with dual antiplatelet therapy consisting of aspirin and a P2Y12 inhibitor, preferably ticagrelor or prasugrel unless contraindicated, for at least 12 months, unless a shortened duration is warranted due to high bleeding risk. Current guidelines also support consideration of low-dose anticoagulation with rivaroxaban in addition to aspirin and clopidogrel, but its use is limited to patients at high ischaemic and low bleeding risk with no prior history of stroke or transient ischaemic attack.36-39 Based on their properties, FXIa inhibitors may, therefore, find a use in certain patients with ACS. PACIFIC-AMI is the only phase II trial to date that has investigated this indication by comparing asundexian at three different once-daily doses (10, 20 or 50 mg) with placebo.40 It had a double-blind, parallel-group design and randomised 1,601 patients up to five days after admission with acute myocardial infarction (AMI) treated with dual antiplatelet therapy to one of the three asundexian doses or placebo. More than 99% of patients underwent percutaneous coronary intervention (PCI) and were randomised after the procedure. Bleeding Academic Research Consortium (BARC) type 2, 3 or 5 bleeding was the main safety outcome and there was no difference in bleeding rates between the combined asundexian and placebo groups (9%). There was also no significant difference in bleeding rates between patients receiving the highest dose of asundexian and those receiving placebo, although rates were higher with increasing doses of asundexian (7.59%, 8.06% and 10.45% for 10, 20 and 50 mg, respectively). There were no fatal bleeding events. Similarly, there was no difference in the primary efficacy outcome of cardiovascular death, AMI, stroke or stent thrombosis between the combined asundexian groups and the placebo group, although the trial was not statistically powered for this. Rates of SAEs were also similar across all groups.
FXI inhibitors for ischaemic stroke
The use of anticoagulants in non-cardioembolic ischaemic stroke is not established and current guidelines recommend long-term single antiplatelet therapy for secondary prevention (sometimes following a short course of dual antiplatelet therapy in specific circumstances).41 However, the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial demonstrated that the combination of low-dose rivaroxaban and aspirin resulted in a lower rate of ischaemic stroke than aspirin monotherapy in patients with a history of stable atherosclerotic vascular disease, excluding patients with high-bleeding-risk conditions.39,42 The lower rates of ischaemic stroke among patients with FXI deficiency have further encouraged the investigation of FXIa inhibitors, in particular for secondary prevention.12,43 Asundexian has been investigated in PACIFIC-STROKE, a randomised, double-blind, parallel-group phase IIb trial and the first of its kind.44 There were 1,808 patients with acute non-cardioembolic ischaemic stroke (within 48 hours of symptom onset) randomised to receive oral asundexian 10, 20 or 50 mg once daily or placebo, in addition to standard antiplatelet therapy. The primary efficacy outcome was a composite of incident covert brain infarcts detected on magnetic resonance imaging and recurrent symptomatic ischaemic stroke at or before 26 weeks. The primary safety outcome was ISTH-defined major or clinically relevant non-major bleeding. There was no significant difference in either of these outcomes between any of the asundexian groups and the placebo group, nor was there any dose-response association. While both primary and secondary efficacy end points were negative, post-hoc analysis did demonstrate a reduction in the composite outcome of recurrent ischaemic stroke and transient ischaemic attack in patients treated with asundexian 50 mg compared with placebo, especially among those with co-existent atherosclerosis.
In the phase II trial AXIOMATIC-SSP, investigators studied the efficacy and safety of various dosage regimens of oral milvexian (25 mg four-times daily and 25, 50, 100 and 200 mg all twice daily) for secondary stroke prevention, in addition to aspirin and clopidogrel. The results of this randomised double-blind trial have not yet been published, but preliminary data presented at the 2022 European Society of Cardiology Congress did not demonstrate a difference in the composite primary efficacy end point of symptomatic ischaemic stroke and covert infarct on magnetic resonance imaging at 90 days between milvexian and placebo. There was an approximately 30% relative risk reduction for symptomatic ischaemic stroke for all dosing regimens of milvexian compared with placebo except for 200 mg twice daily. The incidence of major bleeding (BARC type 3 and 5) was low, but higher in milvexian groups of 50 mg twice daily and above compared with the placebo group.
Upcoming phase III trials
The phase II results have paved the way for phase III trials in patients with AF and patients with ischaemic, non-cardioembolic stroke or high-risk transient ischaemic attack in addition to standard antiplatelet therapy as well as, more speculatively, following ACS (table 3). The results of these studies should help to define the place of FXI/FXIa inhibitors in these conditions. The first phase III trial to start, OCEANIC-AF, has been stopped early due to suggestion of inferior efficacy in the asundexian arm compared with apixaban. Safety data on asundexian are consistent with previous studies. A planned study comparing asundexian with placebo in patients with AF at high risk for stroke or systemic embolism who are deemed ineligible for oral anticoagulation, OCEANIC-AFINA, is being re-evaluated. Other phase III trials investigating other FXI/FXIa inhibitors in AF or asundexian in other clinical settings are ongoing.
Table 3. Upcoming large phase III trials of FXI/FXIa inhibitors
| Trial name | Estimated no. of patients | FXI/FXIa inhibitor | Comparator | Primary efficacy outcome | Estimated maximum follow-up |
|---|---|---|---|---|---|
| Atrial fibrillation | |||||
| OCEANIC-AF* (NCT05643573) | 18,000 | Asundexian | Apixaban | Time to first occurrence of stroke or systemic embolism | 34 months |
| LIBREXIA-AF (NCT05757869) | 15,500 | Milvexian | Apixaban | 48 months | |
| LILAC-TIMI 76 (NCT05712200) | 1,900 (deemed unsuitable for oral anticoagulation) | Abelacimab | Placebo | 30 months | |
| Ischaemic stroke | |||||
| OCEANIC-STROKE (NCT05686070) | 9,300 | Asundexian | Placebo | Time to first occurrence of ischaemic stroke | 31 months |
| LIBREXIA-STROKE (NCT05702034) | 15,000 | Milvexian | Placebo | 41 months | |
| Acute coronary syndrome | |||||
| LIBREXIA-ACS (NCT05754957) | 16,000 | Milvexian | Placebo | Time to first occurrence of major adverse cardiovascular event | 42 months |
| * OCEANIC-AF stopped early due to concerns about inferior efficacy with asundexian compared with apixaban | |||||
Conclusion
Currently used anticoagulants target the common pathway of coagulation, thus, effectively preventing or treating thromboembolic disease at the expense of an increased risk of bleeding. Consequently, targeting the intrinsic pathway of coagulation by suppressing the contribution of FXI or FXII has been the subject of much interest recently. Phase II trials of various FXI/FXIa inhibitors have so far investigated their potential in post-operative VTE prophylaxis, AF, AMI and ischaemic stroke. All agents demonstrated suppression of FXI levels or activity and acceptable tolerance by patients. The four VTE prophylaxis trials collectively showed at least non-inferiority to enoxaparin for both efficacy and safety after knee replacement, with a suggestion of possible superiority.30 However, they were limited by small numbers, exclusion of patients at high bleeding risk and/or superior safety being driven by minor bleeding events.31
None of the phase II trials of asundexian and milvexian were powered to show any difference in primary efficacy outcomes over current standard of care for AF, AMI or ischaemic stroke, although post-hoc analysis in PACIFIC-STROKE suggested potential benefit of asundexian over placebo for reducing the composite of recurrent ischaemic stroke and transient ischaemic attack. Safety profiles of these small-molecule drugs look highly promising, including when combined with antiplatelet therapy or when compared with a DOAC, but phase III trial results are required to more accurately assess their impact on bleeding, as well as determine whether their efficacy matches that of DOACs.
The phase II results have been encouraging and serve as a platform for the development of larger, phase III trials to further investigate their efficacy and safety profiles. Despite early termination of OCEANIC-AF, the safety of FXI/FXIa inhibitors seems to be established and more focus is expected on patients with high bleeding risk or ESRD who would otherwise be unsuitable for current oral anticoagulant therapy.
Key messages
- Factor (F) XI and its activated form FXIa offer attractive targets for novel anticoagulant therapies in view of the role of FXIa in thrombosis and limited contribution to haemostasis
- Genetic studies and phase II trials have provided a foundation for large phase III randomised-controlled trials that will establish the safety and efficacy of FXIa inhibitors across a range of indications
Conflicts of interest
RFS reports institutional research grants/support from AstraZeneca, Cytosorbents, and GlyCardial Diagnostics; and personal fees from Alfasigma, Alnylam, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, Cytosorbents, Daiichi Sankyo, GlyCardial Diagnostics, Idorsia, Intas Pharmaceuticals, Novartis, NovoNordisk, PhaseBio, Sanofi Aventis, and Thromboserin. MAS and NE: none declared.
Funding
MAS and RFS are supported by the National Institute for Health and Care Research (NIHR) Sheffield Biomedical Research Centre (NIHR203321). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
References
1. Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. https://doi.org/10.1016/S0140-6736(13)62343-0
2. Bracey A, Shatila W, Wilson J. Bleeding in patients receiving non-vitamin K oral anticoagulants: clinical trial evidence. Ther Adv Cardiovasc Dis 2018;12:361–80. https://doi.org/10.1177/1753944718801554
3. Jiang H, Jiang Y, Ma H et al. Effects of rivaroxaban and warfarin on the risk of gastrointestinal bleeding and intracranial hemorrhage in patients with atrial fibrillation: systematic review and meta-analysis. Clin Cardiol 2021;44:1208–15. https://doi.org/10.1002/clc.23690
4. Hellfritzsch M, Grove EL, Husted SE et al. Clinical events preceding switching and discontinuation of oral anticoagulant treatment in patients with atrial fibrillation. Europace 2017;19:1091–5. https://doi.org/10.1093/europace/euw241
5. Mohammed BM, Matafonov A, Ivanov I et al. An update on factor XI structure and function. Thromb Res 2018;161:94–105. https://doi.org/10.1016/j.thromres.2017.10.008
6. Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood 2010;115:2569–77. https://doi.org/10.1182/blood-2009-09-199182
7. Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol 2019;39:331–8. https://doi.org/10.1161/ATVBAHA.118.312130
8. Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 2009;23:225–9. https://doi.org/10.1016/j.blre.2009.07.002
9. Hsu C, Hutt E, Bloomfield DM et al. Factor XI inhibition to uncouple thrombosis from hemostasis: JACC review topic of the week. J Am Coll Cardiol 2021;78:625–31. https://doi.org/10.1016/j.jacc.2021.06.010
10. Wheeler AP, Gailani D. The intrinsic pathway of coagulation as a target for antithrombotic therapy. Hematol Oncol Clin North Am 2016;30:1099–114. https://doi.org/10.1016/j.hoc.2016.05.007
11. Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011;118:6963–70. https://doi.org/10.1182/blood-2011-07-368811
12. Preis M, Hirsch J, Kotler A et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood 2017;129:1210–15. https://doi.org/10.1182/blood-2016-09-742262
13. Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost 2009;7(suppl 1):84–7. https://doi.org/10.1111/j.1538-7836.2009.03395.x
14. Buller HR, Bethune C, Bhanot S et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015;372:232–40. https://doi.org/10.1056/NEJMoa1405760
15. Younis HS, Crosby J, Huh JI et al. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood 2012;119:2401–08. https://doi.org/10.1182/blood-2011-10-387134
16. Schaefer M, Buchmueller A, Dittmer F et al. Allosteric inhibition as a new mode of action for BAY 1213790, a neutralizing antibody targeting the activated form of coagulation factor XI. J Mol Biol 2019;431:4817–33. https://doi.org/10.1016/j.jmb.2019.09.008
17. Thomas D, Thelen K, Kraff S et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: first evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost 2019;3:242–53. https://doi.org/10.1002/rth2.12186
18. Koch AW, Schiering N, Melkko S et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood 2019;133:1507–16. https://doi.org/10.1182/blood-2018-10-880849
19. Yi BA, Freedholm D, Widener N et al. Pharmacokinetics and pharmacodynamics of abelacimab (MAA868), a novel dual inhibitor of factor XI and factor XIa. J Thromb Haemost 2022;20:307–15. https://doi.org/10.1111/jth.15577
20. Lorentz CU, Verbout NG, Wallisch M et al. Contact activation inhibitor and factor XI antibody, AB023, produces safe, dose-dependent anticoagulation in a phase 1 first-in-human trial. Arterioscler Thromb Vasc Biol 2019;39:799–809. https://doi.org/10.1161/ATVBAHA.118.312328
21. Lorentz CU, Tucker EI, Verbout NG et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood 2021;138:2173–84. https://doi.org/10.1182/blood.2021011725
22. Perera V, Wang Z, Luettgen J et al. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci 2022;15:330–42. https://doi.org/10.1111/cts.13148
23. Perera V, Abelian G, Li D et al. Single-dose pharmacokinetics of milvexian in participants with mild or moderate hepatic impairment compared with healthy participants. Clin Pharmacokinet 2022;61:857–67. https://doi.org/10.1007/s40262-022-01110-9
24. Thomas D, Kanefendt F, Schwers S et al. First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa. J Thromb Haemost 2021;19:2407–16. https://doi.org/10.1111/jth.15439
25. Kubitza D, Heckmann M, Distler J et al. Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: a randomized phase 1 multiple-dose study. Br J Clin Pharmacol 2022;88:3447–62. https://doi.org/10.1111/bcp.15230
26. Walsh M, Bethune C, Smyth A et al. Phase 2 study of the factor XI antisense inhibitor IONIS-FXIRx in patients with ESRD. Kidney Int Rep 2022;7:200–09. https://doi.org/10.1016/j.ekir.2021.11.011
27. Weitz JI, Bauersachs R, Becker B et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA 2020;323:130–9. https://doi.org/10.1001/jama.2019.20687
28. Verhamme P, Yi BA, Segers A et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med 2021;385:609–17. https://doi.org/10.1056/NEJMoa2105872
29. Weitz JI, Strony J, Ageno W et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med 2021;385:2161–72. https://doi.org/10.1056/NEJMoa2113194
30. Nopp S, Kraemmer D, Ay C. Factor XI inhibitors for prevention and treatment of venous thromboembolism: a review on the rationale and update on current evidence. Front Cardiovasc Med 2022;9:903029. https://doi.org/10.3389/fcvm.2022.903029
31. Galli M, Laborante R, Ortega-Paz L et al. Factor XI inhibitors in early clinical trials: a meta-analysis. Thromb Haemost 2023;123:576–84. https://doi.org/10.1055/a-2043-0346
32. January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. https://doi.org/10.1016/j.jacc.2014.03.022
33. January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. https://doi.org/10.1016/j.jacc.2019.01.011
34. Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612
35. Piccini JP, Caso V, Connolly SJ et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022;399:1383–90. https://doi.org/10.1016/S0140-6736(22)00456-1
36. Mega JL, Braunwald E, Wiviott SD et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. https://doi.org/10.1056/NEJMoa1112277
37. Ibanez B, James S, Agewall S et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. https://doi.org/10.1093/eurheartj/ehx393
38. Collet JP, Thiele H, Barbato E et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–367. https://doi.org/10.1093/eurheartj/ehaa575
39. Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. https://doi.org/10.1056/NEJMoa1709118
40. Rao SV, Kirsch B, Bhatt DL et al. A multicenter, phase 2, randomized, placebo-controlled, double-blind, parallel-group, dose-finding trial of the oral factor XIa inhibitor asundexian to prevent adverse cardiovascular outcomes after acute myocardial infarction. Circulation 2022;146:1196–206. https://doi.org/10.1161/CIRCULATIONAHA.122.061612
41. Kleindorfer DO, Towfighi A, Chaturvedi S et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021;52:e364–e467. https://doi.org/10.1161/STR.0000000000000375
42. Perera KS, Ng KKH, Nayar S et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS trial. JAMA Neurol 2020;77:43–8. https://doi.org/10.1001/jamaneurol.2019.2984
43. Salomon O, Steinberg DM, Koren-Morag N et al. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood 2008;111:4113–17. https://doi.org/10.1182/blood-2007-10-120139
44. Shoamanesh A, Mundl H, Smith EE et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022;400:997–1007. https://doi.org/10.1016/S0140-6736(22)01588-4
45. Perera V, Wang Z, Lubin S et al. Effects of rifampin on the pharmacokinetics and pharmacodynamics of milvexian, a potent, selective, oral small molecule factor XIa inhibitor. Sci Rep 2022;12:22239. https://doi.org/10.1038/s41598-022-25936-2
46. Perera V, Wang Z, Lubin S et al. Effects of itraconazole and diltiazem on the pharmacokinetics and pharmacodynamics of milvexian, a factor XIa inhibitor. Cardiol Ther 2022;11:407–19. https://doi.org/10.1007/s40119-022-00266-6
