Endomyocardial fibrosis is a common cause of restrictive cardiomyopathy worldwide, but rarely occurs in patients living outside tropical regions.1 Herein is the first published case report of a 48-year-old woman with endomyocardial fibrosis due to hypereosinophilia secondary to a rare chromosome 8 and 9 PCM1-JAK2 translocation.
Case summary
A 48-year-old Caucasian woman with no significant past medical history initially presented with a three-week history of progressive exertional dyspnoea and ankle swelling. Systemic enquiry also revealed weight loss of 3 kg over the same time frame, alongside instances of drenching night sweats. The patient was a long-term resident within the United Kingdom with no significant travel history.
Physical examination revealed palpable hepatomegaly alongside pitting oedema to the ankles bilaterally. Examination was otherwise unremarkable with normal heart sounds and jugular venous pressure.
An electrocardiogram demonstrated sinus rhythm with inferolateral T-wave inversion. Serum laboratory tests were remarkable for hypereosinophilia (defined as an eosinophil count >1.5 × 109 cells/L), and bicytopaenia with an elevated N-terminal pro-B-type natriuretic peptide level (table 1).
Table 1. Admission blood test results
| Test | Result | Reference range |
| Eosinophil count | 2.9 × 109 cells/L | 0.02–0.5 × 109 cells/L |
| Haemoglobin | 114 g/L | 120–160 g/L |
| Platelet count | 91 × 109 cells/L | 150–400 × 109 cells/L |
| NT-proBNP | 4,465 ng/L | <400 ng/L |
| Key: NT-proBNP = N-terminal pro-B-type natriuretic peptide | ||
 |
 |
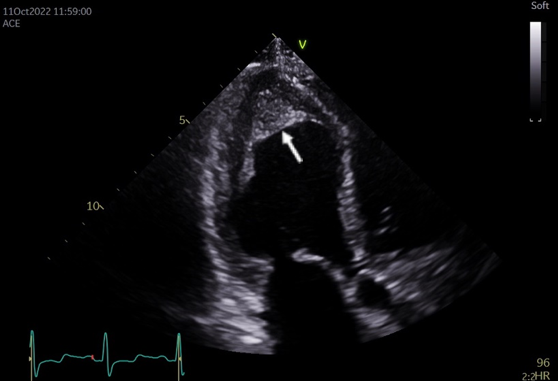
Transthoracic echocardiography (TTE) demonstrated normal left ventricular (LV) size with an ejection fraction of 50–55%. The LV apex was akinetic with obliterating apical myocardial fibrosis and/or a thrombus within the LV apex (figure 1). The right ventricle was dilated with impaired apical function and bi-atrial dilation, moderate mitral regurgitation and severe tricuspid regurgitation were also noted.
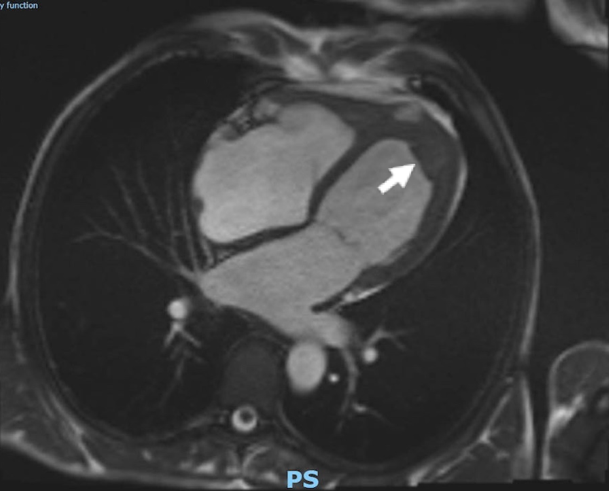
Computed tomography (CT) of the thorax, abdomen and pelvis revealed borderline enlargement of the liver and spleen, but no focal lesion or other abnormality was identified. Cardiac magnetic resonance imaging (MRI) was undertaken to further characterise the LV apical structure and demonstrated endocardial fibrosis with an associated apical thrombus (figure 2).
Following bone marrow aspiration and trephine biopsy, the patient was commenced on high-dose oral prednisolone at 1 mg/kg/day to lower her circulating eosinophil count. Anticoagulation with low-molecular-weight heparin (enoxaparin) at a dose of 1.5 mg/kg once daily for six months was initiated in view of the presence of the LV thrombus, alongside oral bisoprolol 2.5 mg once daily and furosemide 40 mg once daily.
Cytogenetic analysis of bone marrow demonstrated a chromosome 8 and 9 (t(8;9)) translocation of the PCM1 and JAK2 genes suggestive of underlying haematological malignancy. The patient was commenced on ruxolitinib 20 mg twice daily until allogeneic stem cell transplantation.
Discussion
Endomyocardial fibrosis secondary to hypereosinophilic syndrome (HES) is classically demonstrated in the context of helminth or other parasitic infection.2 It is therefore rarely seen in the developed world outside of people who have immigrated from other countries where helminth infection is more common.1 In lifelong residents of Western countries, hypereosinophilia is more likely to be secondary to allergies or medications, or more rarely, haematological or solid malignancies and autoimmune diseases.2
The clinical manifestations of HES are predominantly seen in the skin, lung and gastrointestinal tract in decreasing order of frequency with less than 5% of patients presenting with cardiac manifestations at the onset of disease.3 Cardiac involvement with endomyocardial fibrosis and subsequent thromboembolism is seen to subsequently develop in around 20% of patients.4
The pathophysiology of endomyocardial fibrosis begins with eosinophilic infiltration of the subendocardium. Subsequently, there is predominantly ventricular thrombus formation with risk of embolisation. Over time, subendocardial fibrosis develops secondary to persistent eosinophilic inflammation, risking restrictive cardiomyopathy and valvular disease.5 Arrhythmias can occur due to fibrosis of the conduction system or myocardial scarring.6 Clinical presentation of cardiac eosinophilia therefore differs depending on the stage of myocardial damage, but most commonly presents with dyspnoea and other symptoms of heart failure, with chest pain seen less frequently.6
Myeloid and lymphoid neoplasms with eosinophilia associated with PCM1-JAK2 translocation are rare;7 a literature search did not yield any case reports demonstrating endomyocardial fibrosis in this cohort of patients.
Conclusion
HES causes a wide spectrum of disease, and endomyocardial fibrosis in this context is rarely seen in the developed world. It is important to recognise the possibility of underlying haematological malignancy in this cohort and to explore this within the differential diagnosis of such patients.
Key messages
- Endomyocardial fibrosis secondary to hypereosinophilic syndrome (HES) rarely occurs in patients living outside tropical regions in the absence of parasitic infection
- Hypereosinophilia in the developed world is more likely to be secondary to allergies, medications, or more rarely, haematological or solid malignancies, and autoimmune diseases
- Hypereosinophilia typically manifests with symptoms involving the skin, lung and gastrointestinal tract; cardiac manifestations are rare
- Haematological malignancies should be considered part of the differential diagnosis in HES.
Acknowledgement
HC wrote the article with support from AB. Both authors approved the final submitted version.
Conflicts of interest
None declared.
Funding
None.
Statement of consent
Informed consent was obtained from the patient for publication.
References
1. Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: still a mystery after 60 years. PLoS Negl Trop Dis 2008;2:e97. https://doi.org/10.1371/journal.pntd.0000097
2. Van Balkum M, Kluin-Nelemans H, van Hellemond JJ, van Genderen PJJ, Wismans PJ. Hypereosinophilia: a diagnostic challenge. Neth J Med 2018;76:431–6. https://pubmed.ncbi.nlm.nih.gov/30569889/
3. Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program 2015;2015:92–7. https://doi.org/10.1182/asheducation-2015.1.92
4. Ogbogu PU, Bochner BS, Butterfield JH et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009;124:1319–25.e3. https://doi.org/10.1016/j.jaci.2009.09.022
5. Bondue A, Carpentier C, Roufosse F. Hypereosinophilic syndrome: considerations for the cardiologist. Heart 2022;108:164–71. https://doi.org/10.1136/heartjnl-2020-317202 [Epub online ahead of print]
6. Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart 2016;102:100–6. https://doi.org/10.1136/heartjnl-2015-307959 [Epub online ahead of print]
7. Pozdnyakova O, Orazi A, Kelemen K et al. Myeloid/lymphoid neoplasms associated with eosinophilia and rearrangements of PDGFRA, PDGFRB, or FGFR1 or with PCM1-JAK2. Am J Clin Pathol 2021;155:160–78. https://doi.org/10.1093/ajcp/aqaa208
