Insulin remains an important treatment for patients with type 1 and type 2 diabetes. Insulin is given to patients with type 1 diabetes as a form of hormone replacement therapy to replace the loss of endogenous insulin secretion. Intensive insulin treatment with either continuous subcutaneous insulin infusion or basal–bolus therapy reduces diabetic complications, including macrovascular complications. For patients with type 2 diabetes, insulin therapy is given to try and overcome the combination of insulin resistance and beta-cell dysfunction that are the pathological hallmarks of the disease. There are concerns that weight gain and hypoglycaemia, which are common side-effects of intensive insulin therapy, may reduce or negate direct benefits of controlling hyperglycaemia on macrovascular outcomes. The best insulin regimen for patients with type 2 diabetes is not clear, and treatment should aim to minimise weight gain and the occurrence of hypoglycaemia.
Introduction
 Treatment with insulin is essential for a good prognosis in patients with type 1 diabetes, and it is a potent hypoglycaemic agent in patients with type 2 diabetes. Insulin is an anabolic hormone that influences vascular tone in both large calibre conductance vessels and small calibre exchange vessels such as the capillary microcirculation.1,2 In view of the increased burden of cardiovascular disease in patients with diabetes, and the capability of insulin to influence both metabolic and vascular function, insulin therapy has the potential to improve prognosis from cardiovascular disease in patients with diabetes.
Treatment with insulin is essential for a good prognosis in patients with type 1 diabetes, and it is a potent hypoglycaemic agent in patients with type 2 diabetes. Insulin is an anabolic hormone that influences vascular tone in both large calibre conductance vessels and small calibre exchange vessels such as the capillary microcirculation.1,2 In view of the increased burden of cardiovascular disease in patients with diabetes, and the capability of insulin to influence both metabolic and vascular function, insulin therapy has the potential to improve prognosis from cardiovascular disease in patients with diabetes.
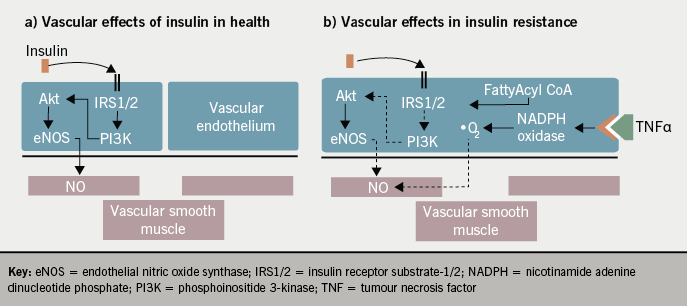
The metabolic effects of insulin are outlined in figure 1. The development of insulin resistance is an independent risk factor for cardiovascular disease and ventricular dysfunction, even prior to the development of type 2 diabetes.3,4 The aetiology of insulin resistance links both metabolic and vascular health. The interaction between adipose tissue and the endothelium in insulin resistance results in decreased endothelial nitric oxide bioavailability.5 This impairs vasodilatation and promotes a prothrombotic state, as well as affecting insulin-mediated capillary recruitment and insulin signalling activity in skeletal muscle as outlined in figure 2. When considering the potential influence of insulin therapy on cardiovascular outcomes it is complicated by the presence of insulin resistance, which is more relevant to those with type 2 diabetes.

Pharmacology
Insulin is a peptide hormone that is given parenterally. There have been many attempts to deliver insulin by the inhaled or oral routes, but at present the route for routine clinical care remains subcutaneous injection. Commercial insulin is produced by recombinant DNA technology with the principle difference between types being the rate of absorption and duration of action. Broadly speaking insulin can be considered as basal insulin (long acting), prandial insulin (short acting) and mixed insulins (a mixture of long and short acting, normally given twice daily). There are now many analogue insulins available. The basal analogues offer the benefit of a flatter dose–response curve, and, thus, a potential for less hypoglycaemia, and the prandial analogues are faster acting and more likely to mimic the normal first-phase insulin response. In patients with type 1 diabetes, the use of analogue insulins allows the attainment of glycaemic targets with less hypoglycaemia. In patients with type 2 diabetes, the use of more expensive analogue insulins slightly reduces the occurrence of hypoglycaemia, but there is no evidence that they improve prognosis.
Trials of safety and efficacy
Type 1 diabetes
The Diabetes Control and Complications Trial (DCCT) demonstrated that microvascular disease (retinopathy, neuropathy and nephropathy) was reduced in patients with type 1 diabetes who had tighter glycaemic control.6 The DCCT population was young (mean age <30 years) with less than 10 years of diabetes duration, resulting in low absolute rates of cardiovascular events, and was, therefore, unable to determine significant macrovascular benefit.7 Seventeen-year follow-up of the DCCT cohorts by the Epidemiology of Diabetes Interventions (EDIC) group showed that the initial period of intensive glycaemic control was subsequently associated with a 42% reduction (p=0.02) in the risk of any cardiovascular event and a 57% reduction (p=0.02) in nonfatal myocardial infarction, stroke or cardiovascular death.8 These benefits were observed after adjustment for risk factors, such as microalbuminuria and dyslipidaemia, and despite abolition of any differences in glycaemic control by the time of the analysis. The cardiovascular benefit of intensive control in type 1 diabetes may be considered to be a ‘legacy’ of tight glycaemic control early in the history of the disease.
Type 2 diabetes
The United Kingdom Prospective Diabetes Study (UKPDS) identified the benefit of microvascular risk reduction in patients with newly diagnosed type 2 diabetes who had intensive therapy with insulin or sulphonylureas, but did not demonstrate any macrovascular benefit during the mean 10 years of the study intervention.9 The UKPDS 10-year Post-Trial Monitoring (UKPDS PTM), totalling a mean of 20 years of follow-up, demonstrated persisting benefits in the patients who had previously been intensively treated (using either sulphonylureas or insulin) with reductions in total mortality (13%, p=0.007), diabetes-related death (17%, p=0.01) and myocardial infarction (15%, p=0.01).10 Initial intervention in UKPDS was in patients with a short duration of diabetes and, as in DCCT/EDIC, differences in glycaemic control were quickly lost after initial study completion. These findings support a similar ‘legacy effect’ in patients with type 2 diabetes as observed in the patients with type 1 diabetes in DCCT/EDIC.
Glycaemic targets
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study, the Veteran Affairs Diabetes Trial (VADT) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) study were designed to assess the impact of tight glycaemic control in older patients with longer duration of type 2 diabetes than those in UKPDS and with a greater burden of established cardiovascular disease (CVD). The ADVANCE study reported no significant reduction in major macrovascular events (p=0.32) or death from any cause (p=0.28).11 The ACCORD intensive treatment group had a higher rate of cardiovascular death (hazard ratio [HR] 1.35, 95% confidence interval [CI] 1.04 to 1.76, p=0.02),12 while the VADT trial did not determine any cardiovascular benefit in intensive glucose lowering.13 These studies are not homogenous. ADVANCE patients had a mean duration of diabetes of eight years at study entry and had generally good glycaemic control, with mean HbA1c at baseline of approximately 7.5% in both conventional and intensive groups. Titration of therapy was gradual, and HbA1c had diverged by 36 months, but compared with ACCORD, less insulin was used.11 In ACCORD, a more aggressive approach to blood glucose management was employed with rapid titration of multiple agents (including insulin) to achieve a mean HbA1c of <7% at four months from a higher baseline of 8.3%.12 VADT participants had poorer baseline control with a median HbA1c at baseline of 9.4% and patients in ACCORD and VADT had longer diabetes duration (10 and 11.5 years, respectively).12,13
Interpreting these studies with a clinical perspective and, in particular, the role of insulin on outcomes is difficult. ACCORD had a lower rate of cardiovascular events than expected, despite the increased risk in the intensive group. Insulin therapy was prominent within the intensive group (77.3% compared with 55.4% in the standard group), however, more than 90% of patients in this group (33% of whom had a previous cardiovascular event at baseline) were prescribed rosiglitazone and had lower than expected use of angiotensin-converting enzyme (ACE) inhibitors, which are important confounders.12 Subgroup analysis of these studies reveals that patients with shorter duration of diabetes, better baseline glycaemic control and no previous CVD exhibit a significant improvement in cardiovascular risk with intensive glycaemic management.14 The trend towards benefit in ADVANCE, and the shorter duration of diabetes in these subjects, might be considered further evidence for the ‘legacy effect’ of previous better glycaemic control. Additionally, deteriorating glycaemic control may be of greater cardiovascular impact in patients with type 1 diabetes than in patients with type 2 diabetes.15 This could make mechanistic sense given that insulin resistance is associated with multiple other metabolic abnormalities, in addition to hyperglycaemia, which promote atherosclerosis.
Meta-analysis of these studies, with the addition of the PROactive study, suggests a cardiovascular benefit of glucose lowering. For five years of treatment with a reduction in HbA1c of 0.9%, significant reductions in nonfatal myocardial infarction (17%) and coronary artery events (15%) would be expected, without a clear signal favouring specific treatments.16
While the specific role of insulin in these intensive glycaemic management studies cannot be separately determined, the role of hypoglycaemia is important to consider. In ACCORD, an episode of severe hypoglycaemia was associated with increased risk of death in either arm of the study.12 It is not considered that this is the cause of excess mortality in the intensive treatment arm since the risk of death was lower in those who had experienced a severe hypoglycaemic event in the intensive arm compared with the standard therapy patients.17 In ADVANCE, hypoglycaemia was a marker for multiple adverse outcomes, including respiratory and digestive disorders, and, in VADT, the strongest predictor of cardiovascular death was a prior severe hypoglycaemic event (HR 4.04, p=0.02).14
This evidence has been considered by both the National Institute for Health and Clinical Excellence (NICE) and the Scottish Intercollegiate Guidelines Network (SIGN), and these groups recommend an HbA1c target of 7.0–7.5% in patients with type 2 diabetes, with tighter control at diagnosis (6.5%). Both guideline groups suggest individual patient assessment and the application of pragmatic limits to glycaemic goals with particular emphasis on avoidance of both hypoglycaemia and weight gain.18,19 These recommendations are consistent with the joint position statement from the American Diabetes Association (ADA), American College of Cardiology Foundation (ACCF) and American Heart Association (AHA).20 There is no evidence from these studies to determine whether insulin per se is of particular benefit or harm with respect to CVD. It is, therefore, more important to select the appropriate patient in which to intensify treatment, than the treatment modality used.
Insulin in acute cardiac disease
Acute coronary syndromes
The Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study was designed to assess the effect of tight metabolic control in the context of acute myocardial infarction. Intensive glucose control using insulin-glucose infusion (aiming for a target blood glucose of 7–10.9 mmol/L) was used for the initial 24 hours of in-patient stay, followed by three months of subcutaneous insulin therapy administered in a basal–bolus regimen. Fewer events occurred than predicted, which may have contributed to the lack of significant difference in mortality up to three months. By one year, a relative reduction in mortality of approximately 30% was observed in the infusion group (p=0.027).21 However, subgroup analysis would suggest that this result is predominantly driven by benefit in only the lowest risk patients (low cardiovascular risk profile, no previous insulin treatment). These observations may reflect the reduced power of the study or, in parallel with observations in Steno-2, that glycaemia is a less important cardiovascular risk factor than lipids, blood pressure or smoking.22 Acute hypoglycaemia was more common in patients receiving insulin infusion, but this did not appear to increase mortality.14 DIGAMI could not determine whether the acute control of glycaemia or the three months of subcutaneous therapy was responsible for the improved one-year mortality.21 DIGAMI 2 set out to clarify this point by including a treatment arm with acute insulin infusion, followed by standard control. DIGAMI 2 was hampered by slow recruitment and ultimately lower-than-expected patient numbers, which reduced the power of the study. The observed improved mortality in the original DIGAMI study was not seen in DIGAMI 2, however, this is explained by a number of factors. First, the overall glycaemic control at baseline in DIGAMI 2 was better than in the original study. Second, apart from a difference in glycaemia in the first 24 hours, prolonged follow-up of all three DIGAMI 2 groups did not show any difference in overall glycaemic control. This in itself is partly due to less aggressive glucose reduction in the intensive groups, and greater use of other hypoglycaemic agents in the control group. Third, event rates were lower than expected, which may, in part, be due to greater use of secondary prevention agents and, in part, due to the underpowering of the trial. Perhaps the most important observation from this study is that blood glucose, either measured by plasma glucose or HbA1c is a significant independent predictor of mortality.23 The similar outcomes in all three groups may suggest that glucose-lowering is of benefit, but that specific insulin use is not proven to be of greater benefit than other strategies. More recent observational data from the Euro Heart Survey would suggest that insulin is associated with a poorer prognosis, however, this is likely to reflect a cohort of patients with poorer glycaemic control, greater underlying insulin resistance and more previous cardiovascular events.24 The Bypass Angioplasty Revascularisation Investigation 2 Diabetes (BARI 2D) study has examined the use of insulin versus insulin-sensitising agents in patients with type 2 diabetes undergoing either percutaneous revascularisation or intensive medical therapy. There was no significant difference in mortality or major cardiovascular events between those treated with insulin or other agents even though insulin therapy was associated with both poorer glycaemic control (HbA1c 7.5% vs. 7.0%, p<0.001) and greater risk of hypoglycaemia (p<0.001).25
Heart failure
In states of physiological stress, the autonomic response is deleterious to cardiac function not simply due to the action of catecholamines inducing increased myocardial work, but also related to increased insulin resistance and hyperglycaemia, which induce further metabolic stress on an effectively energy deficient cardiomyocyte. Despite a logical conclusion that restoration of euglycaemia during heart failure should be metabolically (and subsequently functionally) beneficial, there have been concerns that institution of insulin therapy may exacerbate or even provoke heart failure.26 However, in DIGAMI and DIGAMI 2, there was no observed increase in rates of heart failure in insulin treated groups21,23 and in the Hyperglycaemia: Intensive Insulin Infusion in Infarction (HI-5) study 24 hours of intensive insulin-glucose therapy resulted in lower rates of congestive heart failure.27 Additionally, a case–control study of patients with established type 2 diabetes and heart failure did not reveal any increased mortality in those receiving insulin therapy.28 Insulin resistance is independently associated with left ventricular dysfunction,4 and, since clinically, the most insulin-resistant patients often require exogenous insulin therapy to overcome symptomatic hyperglycaemia, there is an opportunity for insulin therapy to confound potential benefit in heart failure because of its use in more metabolically unwell patients. Effectively, insulin therapy in type 2 diabetes is a surrogate marker for more severe insulin resistance and, therefore, potentially more severe heart failure.
Conclusions
Insulin offers a practical and simple method of achieving rapid glycaemic control in many patients with diabetes. In the context of an acute coronary event it should be considered if the diagnosis of diabetes is new or current glycaemic therapy is inadequate. In patients with type 1 diabetes it is a fundamental therapy that should not be stopped, while, in patients with type 2 diabetes, it is part of an increasing formulary of options for achieving glycaemic control. In the longer term, tight glycaemic control using insulin has proven cardiovascular benefit in patients with type 1 diabetes, and is likely to have benefit in patients with type 2 diabetes, provided the appropriate patient is selected, the speed at which therapy is escalated is not excessive and the target aimed for is not unrealistic and does not represent a dangerous risk of hypoglycaemia. The idea of good control ‘at all costs’ is unrealistic and potentially dangerous, particularly in patients with long-standing type 2 diabetes and especially when it would appear that treatment of other established cardiovascular risk factors represents a better risk–benefit balance.
Conflict of interest
MF has recieved honoraria for lectures and advisory boards from Eli Lilly, Novo Nordisk and Sanofi. NB, GM: none declared.
Key messages
- Intensive insulin treatment in type 1 diabetes significantly reduces diabetic complications, including macrovascular complications
- In type 2 diabetes, insulin therapy is used to overcome the combination of insulin resistance and beta-cell dysfunction with clear benefits of improved outcomes from microvascular and, although less marked, macrovascular complications. Glycaemic targets remain controversial; weight gain and hypoglycaemia should be avoided if possible
- Following acute coronary syndromes intravenous insulin followed by subcutaneous insulin can be used to manage hyperglycaemia
- In patients with chronic heart failure insulin usage is a marker of diabetic patients who have a poor prognosis
References
- Baron AD. Hemodynamic actions of insulin. Am J Physiol 1994;267(2 Pt 1):E187–E202.
- Sydow K, Mondon CE, Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med 2005;10(suppl 1):S35–S43.
- Hu FB, Stampfer MJ, Solomon CG et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 2001;161:1717–23. (doi: 10.1001/archinte.161.14.1717)
- Dinh W, Lankisch M, Nickl W et al. Insulin resistance and glycemic abnormalities are associated with deterioration of left ventricular diastolic function: a cross-sectional study. Cardiovasc Diabetol 2010;9:63. (doi: 10.1186/1475-2840-9-63)
- Serne EH, de Jongh RT, Eringa EC, Ijzerman RG, de Boer MP, Stehouwer CD. Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem 2006;42:163–76. (doi: 10.1042/bse0420163)
- The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86. (doi: 10.1056/NEJM199309303291401)
- Genuth S. Exogenous insulin administration and cardiovascular risk in non-insulin-dependent and insulin-dependent diabetes mellitus. Ann Intern Med 1996;124(1 Pt 2):104–09.
- Nathan DM, Cleary PA, Backlund JY et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. (doi: 10.1056/NEJMoa052187)
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53. (doi: 10.1016/S0140-6736(98)07019-6)
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. (doi: 10.1056/NEJMoa0806470)
- Patel A, MacMahon S, Chalmers J et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. (doi: 10.1056/NEJMoa0802987)
- Gerstein HC, Miller ME, Byington RP et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. (doi: 10.1056/NEJMoa0802743)
- Duckworth W, Abraira C, Moritz T et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. (doi: 10.1056/NEJMoa0808431)
- Schernthaner G. Diabetes and cardiovascular disease: is intensive glucose control beneficial or deadly? Lessons from ACCORD, ADVANCE, VADT, UKPDS, PROactive, and NICE-SUGAR. Wien Med Wochenschr 2010;160:8–19. (doi: 10.1007/s10354-010-0748-7)
- Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 2008;31:714–19. (doi: 10.2337/dc07-2124)
- Ray KK, Seshasai SR, Wijesuriya S et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–72. (doi: 10.1016/S0140-6736(09)60697-8)
- Bonds DE, Miller ME, Bergenstal RM et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. (doi: 10.1136/bmj.b4909)
- National Institute for Health and Clinical Excellence. Type 2 diabetes: the management of type 2 diabetes. NICE clinical guideline 87. London: NICE, 2009.
- Scottish Intercollegiate Guidelines Network. Management of Diabetes. Guideline No. 116. Edinburgh: SIGN, 2010.
- Skyler JS, Bergenstal R, Bonow RO et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009;53:298–304. (doi: 10.1016/j.jacc.2008.10.008)
- Malmberg K, Ryden L, Efendic S et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65. (doi: 10.1016/0735-1097(95)00126-K)
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. (doi: 10.1056/NEJMoa021778)
- Malmberg K, Ryden L, Wedel H et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–61. (doi: 10.1093/eurheartj/ehi199)
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Ryden L. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008;29:177–84. (doi: 10.1093/eurheartj/ehm519)
- Frye RL, August P, Brooks MM et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. (doi: 10.1056/NEJMoa0805796)
- Sheehan JP, Sisam DA, Schumacher OP. Insulin-induced cardiac failure. Am J Med 1985;79:147–8. (doi: 10.1016/0002-9343(85)90562-5)
- Cheung NW, Wong VW, McLean M. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006;29:765–70. (doi: 10.2337/diacare.29.04.06.dc05-1894)
- MacDonald MR, Eurich DT, Majumdar SR et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the UK General Practice Research Database. Diabetes Care 2010;33:1213–18. (doi: 10.2337/dc09-2227)
