Patients with chronic heart failure (CHF) may have low pulse pressures (PP). This retrospective study was undertaken to analyse the relationship between PP and outcomes of a 12-week exercise training programme. Data analysed from 86 patients (69 male) aged 40 to 86 years, included: PP, functional capacity (metabolic equivalents [METS]) and quality of life (QoL) using the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
Median pre-training PP was 54 ± 19 mmHg. Functional capacity for the same heart rate (estimated 60% heart rate reserve) and Borg rating of 13 increased from 3.6 ± 1.1 to 4.0 ± 1.2 METS (p=0.0005); MLHFQ scores improved from 26 ± 19 to 22 ± 20 (p=0.0005). There was a high correlation between PP and systolic blood pressure pre- and post-training (pre: r=0.77, p=0.0005; post: r=0.80, p=0.0005). Changes in all the above outcomes were independent of pre-training PP.
In conclusion, low PP did not reduce the efficacy of an exercise training programme, indicating that CHF patients with low PP can benefit similarly to those with normal/raised PP.
Introduction
Pulse pressure (PP) can be calculated from the difference between the systolic and diastolic blood pressure. With a resting blood pressure of 120/80 mmHg the PP is 40 mmHg. A low PP ≤20 mmHg, in a blood pressure of 90/70 mmHg for example, may represent a decrease in cardiac output1 and reflects a reduction of stroke volume due to left ventricular dysfunction.2
Implications for cardiac rehabilitation (CR)
Wilson et al.3 concluded that patients with chronic heart failure (CHF) and exercise intolerance fall into two general groups. One group includes those who have normal or nearly normal cardiac outputs during exercise and respond well to an exercise training programme, with >10% increase in peak exercise VO2. The other group includes those who exhibit reduced cardiac output responses to exercise. These patients do less well with CR, often finding exercise training exhausting. In 1989, Stevenson and Perloff4 demonstrated that cardiac output can be assessed reliably by PP and that PP can be used to identify the presence of a severely reduced cardiac index rather than to estimate the exact value. In light of this evidence, the aim of this study was to analyse the relationship between PP and outcomes of a
12-week exercise training programme.
Methods
Retrospective data on the changes in the haemodynamic profile and programme outcomes of 86 CHF patients were assessed following a 12-week exercise-based rehabilitation programme. All patients had a diagnosis of left ventricular systolic dysfunction (LVSD) confirmed by echocardiography and were optimised on heart failure medication. Demographic and clinical variables can be seen in table 1. Patients were assessed at their initial CR appointment, and at a separate appointment following completion of the 12-week exercise training programme.

Submaximal exercise tolerance test (SETT)
Patients were assessed before and after the 12-week exercise training programme using either a Chester step test5 or a six-minute walk test.6 Stepping rates and step heights performed, or average six-minute walking paces, were converted into estimated metabolic equivalents (METS).7 Heart rate and blood pressure were recorded before and after SETT. The SETT was stopped if either the heart rate exceeded 59% of heart rate reserve, or the patient reported a rating of perceived exertion (RPE) of >13 or reported symptoms of discomfort such as increased shortness of breath, chest pain or dizziness.
Quality of life (QoL)
Patients completed the Minnesota Living with Heart Failure Questionnaire (MLHFQ) before and after the 12-week programme. The MLHFQ measures the effects of CHF and treatments on an individual’s QoL, and is considered a responsive tool for distinguishing the changes in QoL in CHF patients.8
Blood pressure
Recordings of systolic and diastolic blood pressure were taken before and after SETT, in the supported right or left arm of the seated subject with a cuff-size adjustment based on arm circumference using a Colin Press-mate blood pressure monitor. Resting blood pressure was taken after five minutes of quiet rest, and exercise blood pressure was taken immediately on cessation of the SETT.
Training protocol
Patients attended out-patient supervised exercise training sessions twice per week for 12 weeks. The training programme followed guidelines of the British Association for Cardiac Rehabilitation (BACR)9 and the Association for Chartered Physiotherapists in Cardiac Rehabilitation (ACPICR).10 A 10 to 15 minute warm-up period was followed by 22 minutes of exercise training, comprised of aerobic and low-intensity resistance exercises. The SETT level and Borg RPE scale11 were used to individually prescribe exercise; thus, patients exercised at an intensity range between 40 and 59% of heart rate reserve and/or at an RPE of 12 to 13, as recommended by the ACPICR guidelines.10 Over the 12 weeks, the exercise programme was progressed on the basis of increasing the level of work to always attain these levels. A 10-minute cool-down period completed the exercise session, followed by relaxation.
Statistical analysis
All data were assessed for parametric assumptions prior to statistical analysis using SPSS version 17.0. Data are expressed as mean ± standard deviation (sd) or median ± interquartile range (IQR). No correction has been made for multiple hypothesis testing as this was an exploratory study using retrospective data searching for parameters that will require confirmation in an adequately powered study.
Results
Of the 86 patients, 95% were Caucasian; 69 were male (80%). Fifty-four (63%) had a history of coronary heart disease. The age range was 40 to 86 years, with a mean age of 67 years. In 25 patients (29%) systolic blood pressure failed to increase or dropped slightly (≤15 mmHg) during the SETT. Median PP pre-training was 54 ± 19 mmHg. See table 1 for demographic and clinical variables. See figure 1 for resting PP results.
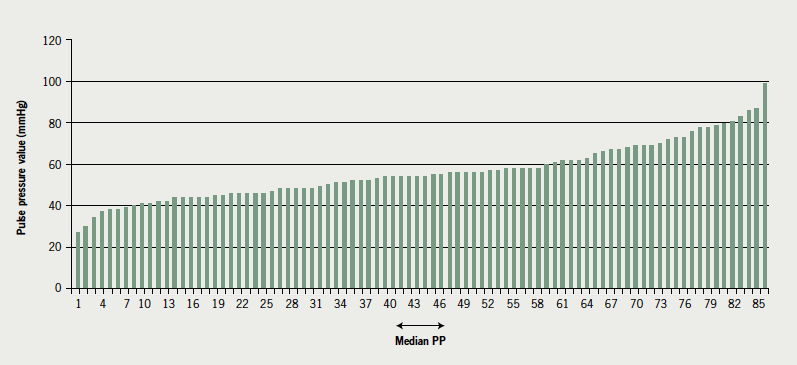
Functional capacity increased from 3.6 ± 1.1 to 4.0 ± 1.2 METS (p=0.0005) and MLHFQ scores improved from 26 ± 19 to 22 ± 20 (p=0.0005) (table 2). There was a significant high correlation12 between PP and systolic blood pressure at both assessment one (pre-CR) (r=0.77, p=0.0005) and at assessment two (post-CR) (r=0.80, p=0.0005) but there was no correlation between PP and diastolic blood pressure at either assessment one or two.

To analyse the effects of low PP on outcomes, criteria for subgroups were set as equal to or less than the median PP value of 54 mmHg (n=44) and above the median PP value of 54 mmHg (n=42). Although there is not an equal number in each group, due to several patients having a PP equal to the median value of 54 mmHg, there is no suggestion that this has biased the results. Patients with a PP below the median value of 54 mmHg had a significantly lower systolic blood pressure (p=0.0005). There were no significant differences between groups in either outcomes post-exercise training (table 2) or in diastolic blood pressure. Figure 2 shows the change in METS against baseline PP.

Discussion
The demographic data reported in this study are similar to those reported by the Framingham Heart Study:13 43% of patients were aged 71 to 80 years; 80% were male; and 54% had an underlying history of coronary heart disease as the main contributing aetiological factor.
Low PP has been reported to be an independent predictor of mortality in patients with decompensated heart failure,14 in patients with non-ischaemic advanced heart failure15 and in patients with symptomatic left ventricular dysfunction post-myocardial infarction.15 Voors et al.16 studied the link between PP and mortality in patients with advanced CHF, and also analysed the relationship between PP and natriuretic peptides. They reported that patients with a PP ≤35 mmHg had a significantly lower survival and those with a PP below the median value of 45 mmHg had increased atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) levels. In spite of the findings of Voors et al.,16 data from this retrospective study indicate that patients with low PP can still benefit from a 12-week cardiac rehabilitation exercise training programme.
Stevenson and Perloff4 demonstrated that PP can be used to identify the presence of a severely reduced cardiac index. Shelton et al.17 have recently reported that, although cardiac index is significantly lower in patients with heart failure compared with controls at rest and during incremental exercise, there is no difference in the absolute increase in cardiac index from rest to a workload of 60 Watts. This indicates that patients with a low PP/reduced cardiac index are able to increase their cardiac output in response to submaximal exercise. Furthermore, it may explain why these patients can still benefit from a course of exercise training.
From the results of this retrospective study it would certainly appear that patients with low PP due to reduced systolic blood pressure/LVSD do as well as patients with normal/raised PP. This group of patients improved in both physical function and their perceived QoL (MLHFQ score). It seems, therefore, that low PP does not limit improvements in exercise capacity and QoL in patients with CHF.
It is important to note that this analysis is exploratory in nature and the data collected are in a clinical setting. It is, therefore, recognised that the findings require verification in prospective studies of appropriate populations. Blood pressure measurement was not performed in a uniform manner. This may be regarded as a criticism, but reflects routine practice.
Acknowledgements
The authors would like to acknowledge the support of the following people: Professor David Cotterall, University of Chester; Professor Keith George, Liverpool John Moores University; Andrew Smallwood, Cardiovascular Research Nurse, New Cross Hospital, Wolverhampton.
Conflict of interest
None declared.
Key messages
- This retrospective study demonstrates that a structured exercise training programme favourably influenced functional capacity and quality of life in patients with a pulse pressure (PP) equal to, below and above the median of 54 mmHg
- A lower PP in this cohort appears to be driven by left ventricular dysfunction (indicated by reduced systolic blood pressure) rather than aortic elasticity (indicated by increased diastolic blood pressure)
- Structured exercise following British Association for Cardiovascular Prevention and Rehabilitation (BACPR) and Association of Chartered Physiotherapists in Cardiac Rehabilitation (ACPICR) guidelines and standards appears to be safe for patients with chronic heart failure in this small retrospective study, regardless of PP
References
- Gopal M, Karnath B. Clinical diagnosis of heart failure. Hospital Physician 2009;45(7):9–15.
- Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk factors for coronary heart disease? The Framingham Heart Study. Circulation 1999;100:354–60.
- Wilson JR, Groves J, Rayos G. Circulatory status and response to cardiac rehabilitation in patients with heart failure. Circulation 1996;94:1567–72.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261:884–8. http://dx.doi.org/10.1001/jama.1989.03420060100040
- Sykes K, Roberts A. The Chester step test – a simple yet effective tool for the prediction of aerobic capacity. Physiotherapy 2004;90:183–8. http://dx.doi.org/10.1016/j.physio.2004.03.008
- Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six- and 12-minute walking tests in respiratory disease. BMJ 1982;284:1607–08. http://dx.doi.org/10.1136/bmj.284.6329.1607
- American College of Sports Medicine. Guidelines for exercise testing and prescription. 7th Edition. Lippincott Williams and Wilkins, 2007.
- Ni H, Toy W, Burgess D, Wise K, Nauman DJ, Crispell K, Hershberger RE. Comparative responsiveness of short-form 12 and Minnesota living with heart failure questionnaire in patients with heart failure. J Cardiac Fail 2000;6:83–91. http://dx.doi.org/10.1054/jcaf.2000.7869
- British Association for Cardiac Rehabilitation Guidelines for Cardiac Rehabilitation. Blackwell Science Ltd, 1995. Reprinted 2000.
- Association of Chartered Physiotherapists in Cardiac Rehabilitation. Standards for the exercise component of the phase III cardiac rehabilitation. ACPICR, 2006.
- Borg GAV. Borg’s rating of perceived exertion and pain scales. IL: Human Kinetics, 1998.
- Cohen L, Holliday M. Practical statistics for students: an introductory text. London: Paul Chapman Publishing, 1996.
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22(4 suppl A):6A–13A. http://dx.doi.org/10.1016/0735-1097(93)90455-A
- Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol 2004;93:785–8. http://dx.doi.org/10.1016/j.amjcard.2003.12.011
- Petrie CJ, Robertson M, Voors AA, van Veldhuisen DJ, Dargie HJ. Abstract 2615: a low pulse pressure predicts mortality in patients with left ventricular dysfunction post myocardial infarction, but only in those with signs and symptoms of heart failure. Circulation 2007;116:II_579.
- Voors AA, Petrie CJ, Petrie MC et al. Low pulse pressure is independently related to elevated natriuretic peptides and increased mortality in advanced chronic heart failure. Eur Heart J 2005;26:1759–64. http://dx.doi.org/10.1093/eurheartj/ehi270
- Shelton RJ, Ingle L, Rigby AS, Witte KK, Cleland JGF, Clark AL. Cardiac output does not limit submaximal exercise capacity in patients with chronic heart failure. Eur J Heart Fail 2010;12:983–9. http://dx.doi.org/10.1093/eurjhf/hfq086
