Chronic kidney disease (CKD) is thought to affect approximately one in 10 people. Patients with CKD have significantly increased cardiovascular morbidity and mortality in comparison with the general population. This is thought to occur from a combination of increased atherosclerotic disease and medial calcification of arterial walls. Vascular calcification (VC) is recognised as an active, cell-mediated process with similarities to osteogenesis. Numerous systemic and local factors have been identified as inhibitors of calcification including fetuin-A, matrix Gla protein and pyrophosphate. There is also increasing evidence that increased serum phosphorus, serum calcium x phosphorus product, and/or calcium load is associated with increased VC.
Current treatment strategies focus on the correction of markers of mineral metabolism bone disease such as phosphate, calcium, parathyroid hormone and vitamin D. The use of agents such as bisphosphonates and cinacalcet show promise, but further data are awaited before their widespread use as a treatment for VC can be advocated. Imaging techniques currently used to assess VC are also discussed. Research into the mechanisms underlying VC are still being investigated and further insight into these mechanisms will lead to the development of therapeutic agents, which could improve cardiovascular outcomes in patients.
Introduction
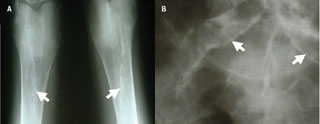
Chronic kidney disease (CKD) is thought to affect approximately one in 10 people and approximately 0.2% of the population has end-stage renal disease (ESRD).1 Patients with CKD have significantly increased cardiovascular morbidity and mortality in comparison with the general population. As CKD progresses towards ESRD the cardiovascular event risk increases.2 This is thought to occur from a combination of increased atherosclerotic disease and increased prevalence of vascular calcification (VC) (figures 1 and 2).

The degree of calcification in coronary arteries is correlated with increased risk of cardiovascular events and death.3 Patients with ESRD have two- to fivefold more coronary artery calcification than age- and sex-matched individuals with coronary artery disease alone.4 VC is highly prevalent in dialysis populations. Studies have estimated that nearly 70% have significant coronary and/or aortic calcification.5
Cardiovascular deaths in the renal population are only partly explained by ischaemic heart disease and myocardial infarction. There is a much higher incidence of arrhythmias, cardiomyopathy and sudden cardiac arrest that cannot be explained by atherosclerosis alone.6 Traditional risk factors cannot explain the increased cardiovascular mortality in this group of patients. This is supported by the fact that the Framingham Risk score has been shown to under predict the cardiovascular event rate in patients with CKD.7
Pathophysiology of VC
Calcification within arteries can take several forms, occurring as very small, dispersed crystals of hydroxyapatite, as large calcified deposits, mineralised cartilage and bone-like tissue containing marrow.8VC is recognised as an active, highly regulated, cell-mediated process with similarities to osteogenesis, in that it is associated with expression of bone-related proteins, such as bone morphogenetic protein-2, osteopontin, type I collagen, bone sialoprotein and alkaline phosphatase.9
In vitro models of calcification have been established, which demonstrate that pericytes, vascular smooth muscle cells (VSMCs) and calcifying vascular cells display many characteristics of cells of the osteoblast lineage and deposit a mineralised matrix reminiscent to that seen in vivo.9,10 Despite intensive investigation, it is still not fully understood how VC is regulated, although matrix Gla protein (MGP), fetuin-A, osteopontin, osteoprotogerin and pyrophosphate are involved.9,11
Inhibitors of VC
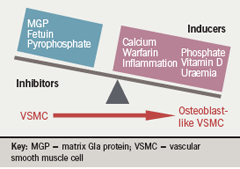
Numerous systemic and local factors have been identified as inhibitors of calcification including fetuin-A, MGP and pyrophosphate. Fetuin-A is a circulating glycoprotein synthesised in the liver. Fetuin-A knockout mice develop extensive vascular and soft-tissue calcification.12 In dialysis patients low circulating fetuin-A levels have been associated with a greater increase in all-cause and cardiovascular mortality.13 MGP-knockout mice develop extensive calcification of arteries and cartilage calcification resulting in premature death despite being normal at birth.14 Mutations in the MGP gene result in human Keutal syndrome (pulmonary stenosis, brachytelephalangism and calcification of cartilage),15 which suggests that MGP is an inhibitor of calcification in humans. MGP requires vitamin K-dependent carboxylation for its function, and treatment with the vitamin K antagonist warfarin results in widespread calcification in rat models.16 Interestingly, warfarin use has been associated with calciphylaxis in CKD patients.17
Pyrophosphate has been known to be an inhibitor of calcium precipitation, and exclusion of pyrophosphate has been demonstrated to be necessary before VC occurs in animal models, even in the presence of hyperphosphataemia and hypercalcaemia (figure 3).18
Relationship between bone and vascular disease
Chronic kidney disease – mineral bone disorder
CKD 4 and 5 patients (i.e. patients with glomerular filtration rate [GFR] <30 ml/min) inevitably develop disorders of mineral metabolism as a consequence of reduced phosphate excretions and reduced vitamin D hydroxylation in the kidney. This results in a compensatory rise in parathyroid hormone (PTH) resulting in hyperparathyroidism. There is increasing evidence that increased serum phosphorus, serum calcium x phosphorus product, and/or calcium load is associated with increased VC.19 Bone density has been shown to be inversely linked to VC in CKD patients.20 VC and renal bone disease are now thought to be part of the same disease spectrum. To that end Kidney Disease: Improving Global Outcomes (KDIGO) have recently published a new definition of renal osteodystrophy termed chronic kidney disease – mineral bone disorder (CKD-MBD). This term is used to describe the clinical syndrome that develops as a systemic disorder of mineral and bone metabolism due to CKD. This syndrome is described as manifesting one or a combination of the following:
- abnormalities of calcium, phosphate, PTH or vitamin D metabolism
- abnormalities in bone turnover, mineralisation, volume, linear growth, or strength
- vascular or other soft tissue calcification.21
VC, calcium, phosphate and PTH
Patients with CKD develop hyperphosphataemia due to reduced phosphate excretion and secondary hyperparathyroidism. Large observational studies have consistently shown an association between hyperphosphataemia and increased cardiovascular outcomes in patients with ESRD and CKD.22,23 Tonelli et al. have also shown that higher serum phosphate is associated with increased cardiovascular events and death in people with pre-existing coronary disease.24
Block et al. studied 40,538 dialysis patients and showed higher adjusted serum calcium concentrations were associated with an increased risk of death. In addition severe hyperparathyroidism (PTH ≥600 pg/ml) was associated with increased mortality.25 Block et al. have also shown that increased calcium x phosphorus products correlate with increased mortality.26 Chertow et al. further added to the association by showing that the use of non-calcium containing phosphate binders attenuated progression of VC in ESRD in comparison with calcium-containing binders, which further increased the calcification present.27
Types of VC
VC can occur in different sites of the vasculature including intimal and medial vessel wall calcification, cardiac valvular calcification and soft tissue calcification.
Intimal calcification
Intimal calcification represents atherosclerotic disease but with increased calcification of the plaques in comparison with the general population.28 It develops mainly in the diabetic or ageing population who have a clinical history of atherosclerotic complications, and is associated with elevated phosphate, lower serum albumin and higher calcium carbonate intake.29 Intimal calcification is observed as a part of advanced atherosclerosis. It is more inflammatory and associated with cholesterol deposition, and usually contributes to arterial stenotic lesions and occlusion of vessels.
Medial calcification

Medial calcification is mainly found in ageing, diabetic and renal populations and is predominantly found in muscle-type conduit arteries such as femoral, tibial and uterine arteries. It is associated with abnormalities in calcium and phosphate metabolism. CKD patients with medial calcification tend to be much younger than patients with intimal calcification, but are also more likely to have a prolonged haemodialysis history (figure 4).29
Calciphylaxis/calcific uraemic arteriolopathy
Calcification can also occur in the medial wall of small vessels and arterioles leading to subsequent tissue necrosis. This is a rare condition in patients with CKD but is associated with significant morbidity and mortality. It has been associated with preceding warfarin use, weight loss and immunosuppressive drugs.17
Valvular calcification
Studies have shown that nearly 50% of haemodialysis patients have evidence of valvular calcification5 and its presence is a marker for atherosclerosis and arterial calcification.30 In comparison with the general population, calcification is markedly increased in mitral (29% vs. 6%) and aortic valves (22% vs. 6%).4
Imaging techniques
Electron beam computed tomography and multi-detector computed tomography
Electron beam computed tomography (CT) and multi-detector CT remain the gold-standard imaging techniques as they provide a quantitative calcification score. Calcification scores strongly predict cardiovascular events in the general population and in dialysis populations.31,32 As a result, these techniques are useful for monitoring the progression of VC. However, these techniques are limited by their ability to differentiate between intimal and medial calcification. Furthermore, cost and availability significantly limit their use in daily practice.
Plain X-ray and ultrasonography
VC can be semi-quantitatively measured using plain X-rays of the lateral lumbar spine. This method has been reported using a scoring system that accounts for the length of calcification on the anterior and posterior section of the aorta corresponding to four consecutive vertebrae. This score has been associated with increased morbidity and mortality in Framingham populations.33 Recently this technique has also been shown to correlate with electron beam CT-generated calcification scores.34
Blacher et al. have shown cardiovascular and all-cause mortality is predicted by the presence and extent of VC using B-mode ultrasonography at the carotid artery, aorta and femoral artery. This method has the advantage that it is more easily performed, cheaper and involves less radiation exposure for the patient.35
While ultrasound-based methods and plain X-rays may be good initial investigations they lack sensitivity and do not allow for a quantitative assessment of calcification load. Therefore, electron beam CT or multi-detector spiral CT should be used to quantify and assess time-related alterations in VC score.
Vascular stiffness
Pulse wave velocity

Vascular stiffness can be used as a surrogate marker of VC that can be measured at the bedside. Normally blood vessels accommodate a large proportion of the blood volume by distending, allowing a smoother delivery of blood to the peripheries. As calcification (intimal or medial) develops it restricts the distensibility of the blood vessels, hence increasing their ‘stiffness’. The gold-standard measurement is carotid-femoral pulse wave velocity. This measures the delay in propagation of the aortic pressure wave from the contraction of the left ventricle to the peripheral vessel (carotid artery and the femoral artery). Having measured the distance between the two points it is possible to determine the speed of the pressure wave through the main vascular tree. The procedure is easily applicable at the bedside though currently it is mainly used in the research setting (figure 5).
Greater pulse wave velocity, and by implication, vascular stiffness, have been associated with increased mortality in dialysis patients and are highly correlated with VC.36
Management of VC
Non-calcium containing phosphate binders
The use of calcium-containing phosphate binders has been associated with increased progression of VC.29 The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines now state that a maximum dose of 1.5 g of elemental calcium should be given per day.37 The newer non-calcium containing phosphate binder (sevelamer hydrochloride) has been shown to attenuate the progression of coronary and aortic calcification when compared with calcium-containing phosphate binders.27 At present the use of non-calcium containing binders is recommended in patients with evidence of VC.38
Vitamin D analogues
There have been mixed data published regarding the association of vitamin D analogues and VC. Data from experimental in vivo and in vitro models have shown vitamin D analogues39 to be associated with increased calcification, though some experiments used higher than physiological doses. However, in comparison retrospective survival analyses have been undertaken and these have shown that patients have a survival advantage if they are taking a vitamin D analogue compared with no vitamin D.40 Traditional vitamin D analogues result in increased calcium and phosphate levels. However, the newer vitamin D analogues do not lead to as great an increase in calcium and phosphate. This is due to selective receptor activation. One hypothesis suggests that vitamin D induced calcification is not due to the direct actions of vitamin D. Instead it is a result of increased episodes of hypercalcaemia and hyperphosphataemia. The newer analogues may help prevent this but no clear evidence is available in patients and further randomised controlled trials are required.
Calcimimetics
Cinacalcet is a calcimimetic that modulates the calcium sensing receptor at the parathyroid gland, and is used for treatment of severe CKD associated hyperparathyroidism. Cinacalcet controls PTH levels while reducing calcium, phosphate and calcium x phosphate product.41 Interestingly Cunningham et al. have shown that patients receiving calcimimetics had reduced hospitalisation from cardiovascular events in comparison with patients with uncontrolled secondary hyperparathyroidism.42 Whether this effect is a result of vascular remodelling secondary to the presence of the calcium sensing receptor in other cells or improvement in hyperparathyroidism is not yet clear.
Bisphosphonates
The bisphosphonate group of drugs are pyrophosphate analogous and have been reported as being inhibitors of vascular and soft tissue calcification in animal models since the 1960s.43 Recent data from small clinical studies have demonstrated that etidronate can arrest or even reduce VC in haemodialysis patients.44 However, it is important to note that bisphosphonates are renally excreted and their use in dialysis patients may be associated with increased side effects. Furthermore, some groups of renal patients have a dynamic bone disease or mixed bone disease, and bisphosphonate treatment may have adverse effects on bone health. Consequently, the routine use of bisphosphonates is not advised at present. Larger randomised controlled trials are awaited that investigate bone and vascular health in addition to clinical outcomes.
Current guidelines for management
Patients with CKD have increased cardiovascular morbidity that is associated with increased VC and increased atherosclerotic disease. Therefore, traditional risk factors for cardiovascular disease should be addressed including smoking cessation, weight loss, glycaemic control and hypertension. In CKD it now appears that management or prevention of VC is closely related to the management of bone disease as highlighted by the new KDIGO definition. As a result, management of mineral and bone disease may reduce cardiovascular risk and the early identification and correction of calcium, phosphate and PTH abnormalities is essential. The KDOQI cardiovascular guidelines have provided recommendations on the management of VC. If calcification is present at two or more sites, patients should be treated with non-calcium containing phosphate binders in addition to aggressive management of traditional and non-traditional risk factors.38
Conclusion
Patients with CKD have increased cardiovascular mortality and morbidity that cannot be attributed to traditional risk factors alone. In particular, VC has been shown to be associated with increased cardiovascular mortality in patients with ESRD. The development of VC is most likely related to an imbalance of local and systemic inhibitors and inducers of calcification. In particular, disorders of mineral metabolism and their treatments have been linked to VC in CKD patients. In addition to intimal and medial calcification, CKD patients can also develop rare life-threatening complications such as calciphylaxis. Current treatment strategies focus on the correction of markers of CKD-MBD such as phosphate, calcium, PTH and vitamin D. In patients with established VC the use of non-calcium containing binders has been recommended. The use of agents such as bisphosphonates and cinacalcet show promise in some groups but further data are awaited before their widespread use as a treatment for VC can be advocated. Research into the mechanisms underlying VC is ongoing, and hopefully further insight into these mechanisms will lead to the development of therapeutic agents that can improve cardiovascular outcomes in patients.
Acknowledgements
Figures 1 and 2 are reproduced courtesy of Dr David Goldsmith, Guys Hospital, London.
Conflict of interest
PAK is on the advisory board for Genzyme and Abbott, has received an educational grant from Amgen and speaker’s fees from Genzyme. SS has received speaker’s fees from Novartis and Shire Pharmaceuticals. HE has received speaker’s fees from Shire Pharmaceuticals.
Key messages
- Vascular calcification is significantly increased in patients with renal dysfunction
- Chronic kidney disease – mineral bone disorder (CKD-MBD) is used to describe the clinical syndrome that develops as a systemic disorder of mineral and bone metabolism due to CKD
- Electron beam CT and multi-detector CT are the gold-standard imaging techniques
- Management focuses on the correction of markers of mineral metabolism
- There are no targeted treatments available for vascular calcification at present
References
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305.
- Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 2001;104:412–17.
- Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis1996;27:394–401.
- Hujairi NM, Afzali B, Goldsmith DJ. Cardiac calcification in renal patients: what we do and don’t know. Am J Kidney Dis 2004;43:234–43.
- Querfeld U. Is atherosclerosis accelerated in young patients with end-stage renal disease? The contribution of paediatric nephrology. Nephrol Dial Transplant 2002;17:719–22.
- Weiner DE, Tighiouart H, Elsayed EF et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 2007;50:217–24.
- Jeziorska M, McCollum C, Wooley DE. Observations on bone formation and remodelling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch 1998;433:559–65.
- Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 2004;95:560–7.
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res 1998;13:828–38.
- Tintut Y, Abedin M, Cho J, Choe A, Lim J, Demer LL. Regulation of RANKL-induced osteoclastic differentiation by vascular cells. J Mol Cell Cardiol 2005;39:389–93.
- Schafer C, Heiss A, Schwarz A et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003;112:357–66.
- Hermans MMH, Brandenburg V, Ketteler M et al. Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 2007;72:202–07.
- Luo G, Ducy P, McKee MD et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78–81.
- Munroe PB, Olgunturk RO, Fryns JP et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet 1999;21:142–4.
- Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 1998;18:1400–07.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial 2002;15:172–86.
- Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 2004;15:2959–64.
- Goodman WG, Goldin J, Kuizon BD et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478–83.
- London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 2004;15:1943–51.
- Moe S, Drueke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53.
- Kestenbaum B, Sampson JN, Rudser KD et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005;16:520–8.
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001;12:2131–8.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112:2627–33.
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208–18.
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998;31:607–17.
- Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62:245–52.
- Schwarz U, Buzello M, Ritz E et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000;15:218–23.
- London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003;18:1731–40.
- Wang AY, Ho SS, Wang M et al. Cardiac valvular calcification as a marker of atherosclerosis and arterial calcification in end-stage renal disease. Arch Intern Med 2005;165:327–32.
- Matsuoka M, Iseki K, Tamashiro M et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol 2004;8:54–8.
- Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826–33.
- Wilson PW, Kauppila LI, O’Donnell CJ et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001;103:1529–34.
- Bellasi A, Ferramosca E, Muntner P et al. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 2006;70:1623–8.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001;38:938–42.
- Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2007;2:1241–8.
- National Kidney Foundation. K/DOQI clinical practice guidelines: bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(suppl 4):S1–S201.
- K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005;45(4 suppl 3):S1–S153.
- Tukaj C, Kubasik-Juraniec J, Kraszpulski M. Morphological changes of aortal smooth muscle cells exposed to calcitriol in culture. Med Sci Monit 2000;6:668–74.
- Teng M, Wolf M, Ofsthun MN et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol 2005;16:1115–25.
- Block GA, Martin KJ, de Francisco AL et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004;350:1516–25.
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 2005;68:1793–800.
- Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev 1998;19:80–100.
- Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial 2006;10:59–64.
