The 2008 American College of Cardiology meeting was held jointly with the Society for Cardiac Angiography and Interventions Annual Meeting on March 29th – April 1st in Chicago, US. Highlights of the meeting included discussions about the ENHANCE trial which showed a shock negative result for the cholesterol agent, ezetimibe; and studies showing that hypertension should be treated in the over-80s, that a combination of a calcium blocker and an ACE inhibitor is a good first-line treatment for hypertension, and that telmisartan is as effective as ramipril for high risk coronary heart disease/diabetes patients.
ENHANCE controversy dominates meeting
The controversial ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolaemia Enhances Atherosclerosis Regression) trial of ezetimibe dominated the ACC meeting, with a special session held to discuss the results, which were simultaneously published in the New England Journal of Medicine.
The 720-patient trial showed no benefit of ezetimibe when added to simvastatin 80 mg in slowing the progression of atherosclerosis in the carotid artery (as measured by intima media thickness (IMT) in patients with familial hypercholesterolemia (FH), despite the fact that there were significantly greater reductions in low-density lipoprotein (LDL) cholesterol and C-reactive protein in the ezetimibe group.
These ‘bottom line’ results were first announced in a press release in January, and have attracted an enormous amount of attention, probably because they are the first real data on ezetimibe, which has been generating worldwide sales of around US$5 billion for Merck and Schering Plough. In addition, concerns have been raised about the announcement of the results being delayed by up to 18 months, which is now the focus of a US congressional investigation.
Leading the panel discussion on the ENHANCE trial at the ACC, Dr Harlan Krumholz (Yale University School of Medicine, US) recommended that ezetimibe should only be used as a last resort, until further data become available. “We must return to first principles, optimising the doses of statins, rather than adding ezetimibe to a low-dose statin,” he said.
The same advice was given by the ACC in a statement issued during the meeting. “The study reinforces the need to adhere to current American College of Cardiology/American Heart Association Guidelines which recommend statins to the maximally tolerated dose or to goal as first line treatment for patients with coronary artery disease,” the statement read.
Noting that ezetimibe actually showed a trend towards a worse result than simvastatin alone in ENHANCE, Dr Krumholz said there was still a question mark over the drug’s safety. “We don’t know what this drug does, and although it might prove to be effective in reducing clinical events, the ENHANCE findings make this unlikely,” he said.
In a press conference, lead investigator of the ENHANCE trial, Dr John Kastelein (Academic Medical Center, Amsterdam, The Netherlands) said he would still be treating his patients with FH with ezetimibe on top of highest dose statin therapy until more evidence is available as “this is the only way to get their LDL cholesterol down to normal levels”.
In the paper announcing the results (N Engl J Med 2008; 358:1431–43), Dr Kastelein and his co-authors suggest that the reason ezetimibe failed to show a benefit in ENHANCE may be because most of the patients included had been treated with high-dose statin therapy from an early age, “raising the possibility that there may be limits to the extent to which the lowering of LDL cholesterol levels can result in a further decrease in the progression of intima-media thickness”.
In an accompanying editorial, US cholesterol experts Drs Greg Brown (University of Washington School of Medicine, Seattle, US) and Allen Taylor (Walter Reed Army Medical Center, Washington DC, US) concur that this could be a possibility. Noting that patients in ENHANCE had thinner carotid IMT than in previous studies, they say this supports the hypothesis that previous plaque lipid depletion (brought about by long-term statin therapy) is an explanation for the ENHANCE results. The argument against this hypothesis is that no benefit of ezetimibe was seen in the 19% of patients who were not receiving statins at the start of the ENHANCE trial.
Drs Brown and Taylor add that the possibility that ezetimibe provides no clinical benefit when added to statin therapy is the “elephant in the parlor” and deserves serious consideration in any discussion of the study results.
The effect of ezetimibe on clinical events is being investigated in IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial), which is comparing simvastatin 40 mg plus ezetimibe 10 mg with simvastatin 40 mg alone in acute coronary syndrome patients. This trial was originally planned to enrol 10,000 patients but because of low event rates it has recently been extended to 18,000 patients, and results will not be available until 2012.
ONTARGET: telmisartan similar to ramipril in vascular disease/diabetes patients
The angiotensin receptor blocker (ARB), telmisartan, showed similar benefits to the angiotensin-converting enzyme (ACE) inhibitor, ramipril, in patients with vascular disease or high-risk diabetes in ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). But the combination of the two drugs was associated with more adverse events without any additional benefits.
Lead investigator of the trial, Dr Salim Yusuf (McMaster University, Hamilton, Canada) commented: “This study is of clinical importance because it demonstrates that telmisartan is an effective and safe alternative to ramipril. This means both patients and physicians have choices and can use telmisartan where appropriate with a high degree of confidence”. But he added that: “the lack of additional benefit of the combination despite a substantial lowering of blood pressure is puzzling”.
The ONTARGET trial enrolled 25,620 patients with coronary heart disease or diabetes plus additional risk factors who were randomised to ramipril 10 mg per day, telmisartan 80 mg a day or the combination of the two. The mean duration of follow-up of the study was 55 months.
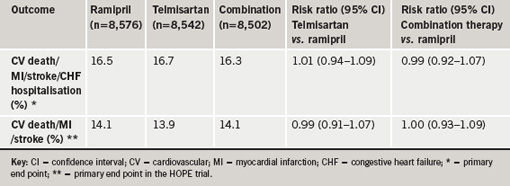
Results showed that mean blood pressure was lower in the telmisartan group (a 0.9/0.6 mmHg greater reduction) and the combination therapy group (a 2.4/1.4 mmHg greater reduction) than in the ramipril group. At the end of the study, the primary end point (a composite of cardiovascular death, myocardial infarction, stroke or hospitalisation for heart failure) had occurred in a similar amount of patients in all three groups (table 1). Compared with the ramipril group, telmisartan patients had lower rates of cough and angioedema, and a higher rate of hypotensive symptoms, and patients given the combination treatment had higher rates of hypotensive symptoms, syncope, renal dysfunction, and hyperkalemia, with a trend towards an increased risk of renal function requiring dialysis (table 2).
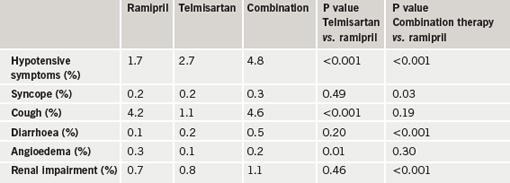
The ONTARGET results were published in the New England Journal of Medicine (N Engl J Med 2008;358:1547–59) to co-incide with their presentation at the ACC. In an accompanying editorial, Dr John McMurray (University of Glasgow) says that because ARBs are more costly than ACE inhibitors and are not better tolerated overall, their primary value is as an alternative for patients who cannot tolerate ACE inhibitors because of cough.
HYVET: treat hypertension in the very elderly
Treatment of hypertension in the very elderly is beneficial, reducing fatal strokes, cardiovascular events and all-cause mortality, results from HYVET (Hypertension in the Very Elderly Trial) showed.
“Before our study, doctors were unsure about whether very elderly people with high blood pressure could see the same benefits from treatment to lower their blood pressure as those we see in younger people,” said Emeritus Professor Christopher Bulpitt (Imperial College London). “Our results clearly show that many patients aged 80 and over could benefit greatly from treatment. Populations are living longer and we have growing numbers of people living well into their 80s and beyond, so this is good news.”
The HYVET trial randomised 3,845 patients aged 80 or over with a systolic blood pressure of at least 160 mmHg to treatment with placebo or indapamide (SR) 1.5 mg with the addition of perindopril 2–4 mg once a day as required, to reach a target blood pressure of 150/80 mmHg. The primary end point was all strokes.
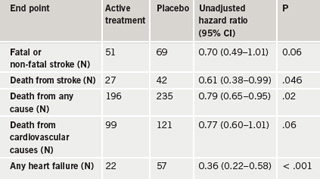
The average follow-up was just over two years, by which time 20% of the placebo group and 48% of the active treatment group had achieved the target pressure. In July 2007, the trial was stopped early after a second planned interim analysis showed a significant reduction in total mortality. Results also showed reductions in death from stroke, and in the occurrence of heart failure (table 1). Fewer adverse side effects were seen in the active treatment group than in the placebo group.
In an editorial accompanying publication of the study in the New England Journal of Medicine (N Engl J Med 2008;358:1958–60), Dr John Kostis (University of Medicine and Dentistry of New Jersey, US), says that “HYVET puts the question of the usefulness of treating hypertension in the very old to rest”.
Noting that current practice has been based on incomplete evidence and speculation, Dr Kostis concludes that “the HYVET investigators deserve to be congratulated for persevering and ultimately succeeding in proving the benefit of treating hypertension in the very old.”
Sponsored by Imperial College London, HYVET was the largest randomised, controlled trial assessing the benefit-to-risk ratio of treating hypertensive patients 80 years of age and older. The results showed that lowering blood pressure of elderly patients could cut their total mortality by a fifth and their rate of cardiovascular events by a third. The findings have the potential to affect the treatment and impact the health outcomes of millions of individuals, the investigators said.
ACCOMPLISH: calcium blocker beats diuretic for combination with ACE inhibitor
The combination of a calcium channel blocker and an angiotensin-converting enzyme (ACE) inhibitor is more effective for reducing cardiovascular events in the treatment of hypertension than a thiazide diuretic plus an ACE inhibitor, the results of the ACCOMPLISH (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension) trial show. Experts say these results should lead to a change in the hypertension guidelines.
The Novartis-funded trial was the first study to evaluate cardiovascular outcomes using single pill, fixed-dose combination therapy for the treatment of high-risk hypertensive patients. It randomised 10,700 patients to a combination of benazepril and amlodipine or to a combination of benazepril and hydrochlorothiazide. All patients in the study received no more than 40 mg of benazepril in each dose; amlodipine doses began at 5 mg and could be increased to 10 mg, while hydrochlorothiazide doses began at 12.5 mg and could be increased to 25 mg.
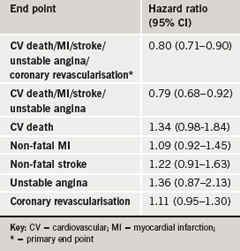
Only 37 % of the participants had adequate control of blood pressure prior to entry into the trial but use of the combination tablets more than doubled the number of patients who achieved adequate control to 80 % in both groups. Although blood pressure was lowered to a similar extent in both treatment arms, the calcium blocker/ACE inhibitor reduced cardiovascular morbidity and mortality by 20% compared to the diuretic/ACEinhibitor group. The study was stopped early because of a lower event rate in the ACE inhibitor/calcium blocker group. Results are summarised in table 1.
Lead investigator, Dr Kenneth Jamerson (University of Michigan, US) said: “The ACCOMPLISH trial demonstrates the superiority of an ACE inhibitor/calcium blocker fixed-dose combination treatment strategy for reducing cardiovascular morbidity and mortality and provides evidence to modify future hypertension guidelines especially in terms of starting with a one-drug strategy.”
“These ACCOMPLISH results shake the foundations of current recommendations and define a new standard which will enhance the achievement of the primary goal and assist clinicians in meeting the daily challenges of hypertension management,” co-investigator Dr Eric Velazquez (Duke University Medical Center, Durham, US) added.
ISAR-REACT 3: bivalirudin in low-risk PCI?
Bivalirudin does not improve net clinical benefit in troponin-negative patients undergoing elective angioplasty (PCI) who have received a 600 mg pre-loading dose of clopidogrel, according to the results of the ISAR-REACT 3 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 3) trial.
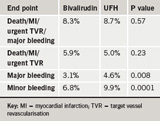
The trial included 4,570 such patients randomised to bivalirudin or heparin. Results showed no significant difference in the primary end point (a composite of death, myocardial infarction [MI], urgent target vessel revascularisation [TVR] or major bleeding at 30 days) or the secondary end point (a composite of death, MI, or urgent target vessel revascularisation) between the two groups, but bleeding was reduced with bivalirudin (table 1).
Presenting the study, Dr Adnan Kastrati (Deutsches Herzzentrum, Munich, Germany) said there was no good reason to use bivalirudin instead of heparin in elective PCI. But discussant, Dr Harvey White (Green Lane Hospital, Auckland, New Zealand), said the reduced bleeding alone was a reason to use bivalirudin. “Bleeding is important to patients in terms of patient discomfort, prolonged hospitalisations, increased costs, and it is independently associated with early and long term morbidity and mortality,” he said.
Paclitaxel coated balloon for in-stent restenosis?
A balloon coated with paclitaxel showed better results in the treatment of in-stent restenosis than using a paclitaxel-eluting stent in the PEPCAD-2 ISR (Paclitaxel-Eluting PTCA-Balloon Catheter in Coronary Artery Disease-2 In-Stent Restenosis) trial.
The primary end point, lumen loss six months after the procedure, was reduced by about half after treatment with the SeQuent™-Please balloon catheter in the “as treated” analysis of the results, which also showed reductions in target-lesion revascularisation rates and major cardiac events, lead investigator Dr Martin Unverdorben (University of Frankfurt, Germany) reported.
But discussant, Dr Steven R Bailey (University of Texas Health Sciences Center, San Antonio, US) noted that the intention to treat analysis did not show significant differences, and the 131-patient study was too small to reach definitive conclusions.
STRADIVARIUS: mixed results for rimonabant on atherosclerosis
The weight loss drug, rimonabant, did not slow the progression of atherosclerosis and was associated with an increased rate of psychiatric (mainly anxiety and depression) and other side effects in STRADIVARIUS (Strategy to Reduce Atherosclerosis Development Involving Administration of Rimonabant – The Intravascular Ultrasound Study). However, total atheroma volume, a secondary end point, was reduced with the drug, and lead investigator, Dr Steven Nissen (Cleveland Clinic, Cleveland, US) concluded that rimonabant still holds promise in heart disease.
The STRADIVARIUS study involved 839 patients with coronary artery disease and abdominal obesity who received either rimonabant 20 mg daily or placebo for 18 months. Atherosclerosis was measured by intravascular ultrasound at the start and end of the study.
Results showed that patients on rimonabant lost an average of 9.5 pounds (4.3kg) and 1.8 inches (4.5 cm) in waist circumference compared with the placebo group. They also had greater improvements in lipid profiles, including a 22.4% increase in high-density lipoprotein (HDL) cholesterol and a 20.5% reduction in triglycerides, and in C-reactive protein and glycosylated haemoglobin (HbA1c).
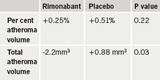
The primary end point, per cent atheroma volume, was no different between the two groups, although the secondary end point of normalised total atheroma volume did show an improvement in the rimonabant patients (table 1).
Psychiatric adverse effects occurred in 43.4% of rimonabant patients versus 28.4% of the placebo group, but Dr Nissen noted that severe psychiatric adverse effects, including major depression and suicidal ideation, were uncommon occurring with similar frequency in the two groups (4.7% on rimonabant and 3.8% on placebo). In addition to psychiatric side effects, rimonabant was also associated with increases in nausea, other gastrointestinal side effects and erectile dysfunction.
In an editorial accompanying publication of the study in JAMA (JAMA 2008; 299: 1547–60) Drs John Rumsfeld (Denver Veterans Affairs Medical Center, Denver, US) and Brahmajee Nallamothu (University of Michigan, Ann Arbor, US) , say the rate of psychiatric adverse events in this study was “alarmingly high”, adding that “given the known correlation between obesity and psychiatric comorbidity, the potential for a medication that targets weight loss to cause depression is a major concern”.
Dr Nissen noted that more information on the effect of rimonabant on heart disease will come from the long-term placebo-controlled outcomes trial, CRESCENDO, which is underway in 17,000 patients at high risk for cardiovascular events.
Valve clip improves mitral regurgitation
Percutaneous mitral repair with a valve clip reduced mitral regurgitation and improved left ventricular function in EVEREST (Endovascular Valve Edge-to-Edge Repair Study).
Presenting the results, Dr James Hermiller (St Vincent Heart Center, Indianapolis, US) noted that 19 of 23 patients had been successfully treated with the clip device. One-year results, available for 12 patients, showed that mitral regurgitation had been reduced to mild or trivial levels in 89% of the patients, and that heart failure functional class and measures of reverse remodelling also showed significant improvement.
If further studies confirm the safety and efficacy of the device, it may prove to be a valuable alternative to surgical repair, enabling patients to avoid open-heart surgery, Dr Hermiller concluded.
Celecoxib meta-analysis will help direct prescribing
A new meta-analysis of trials of the COX-2 inhibitor, celecoxib, has suggested that the drug has the greatest risk of adverse effects when used in patients at highest cardiovascular risk, and that the cardiovascular adverse events appear to be dose related.
Presenting the study, Dr Scott Solomon (Brigham and Women’s Hospital, Boston, US) said the results “should provide comfort in prescribing celecoxib to patients with very low cardiovascular risk, but caution in prescribing the drug in those at high risk”. He added that the data support recent recommendations that physicians should prescribe the lowest doses of celecoxib possible, especially in higher risk patients.
The meta-analysis included six trials that compared celecoxib to placebo for conditions other than arthritis, with a planned follow-up of three or more years. All had been stopped after the withdrawal of rofecoxib in 2004. In total, 7,950 patients were administered one of three doses of celecoxib (400 mg once daily, 200 mg twice daily or 400 mg twice daily). The primary end point was the combination of cardiovascular death, myocardial infarction, stroke, heart failure or thromboembolic event. This was found to be increased with celecoxib in the overall population, but showed a dose-related effect.
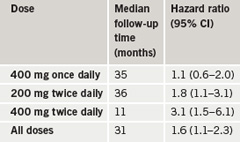
The results (table 1) also showed a doubling of risk between low and moderate cardiovascular risk groups and a further doubling of risk between moderate and high risk groups.
Dr Solomon noted that the studies included in this meta-analysis did not include the most widely used dose of celecoxib for osteoarthritis (200 mg once daily), but rather related to doses recommended for other indications such as rheumatoid arthritis, acute pain and dysmenorrhoea. He suggested that once-daily dosing appears to be safer than twice-daily dosing, but added that it cannot be addressed from this study whether doses lower than those used in these six trials are safer.
PERISCOPE: pioglitazone reduces atherosclerosis progression
Pioglitazone reduced the progression of atherosclerosis and improved cardiovascular risk compared with glimepiride in the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study in patients with type 2 diabetes.
Presenting the results, Dr Steven Nissen (Cleveland Clinic, Cleveland, US) said this was the first time a diabetes drug had been shown to slow progression of coronary atherosclerosis.
In the trial, 543 patients with type 2 diabetes were randomised to either glimepiride (1–4 mg) or pioglitazone (15–45 mg) for 18 months. Atherosclerosis was assessed by intravascular ultrasound at the start and the end of the treatment period. Results showed that mean per cent atheroma volume decreased by 0.16% in pioglitazone-treated subjects but increased by 0.73% in glimepiride-treated patients. Pioglitazone also had greater effects on lowering glycosylated haemoglobin (HbA1c) and fasting insulin levels, and improved high-density lipoprotein cholesterol and triglyceride levels.
In terms of adverse events, more patients in the glimepiride group experienced hypoglycaemia and angina, while oedema, weight gain and bone fractures were more common with pioglitazone.
Dr Nissen cautioned that this study was based on a surrogate end point, and should not influence clinical practice on its own, but that together with the PROACTIVE clinical end point trial, which showed promising results for pioglitazone, “the totality of information suggests this is a beneficial therapy”.
And what about rosiglitazone?
A smaller study (VICTORY) did not show a significant effect of rosiglitazone versus placebo on slowing atherosclerosis progression, but there was a beneficial trend. The 193-patient study also showed a positive effect on metabolic factors with the drug. Lead investigator Dr Olivier Bertrand (Laval University, Quebec, Canada) said the results provided reassurance about the safety of rosiglitazone.
ALLAY: aliskiren as effective as losartan for regressing LVH
The direct renin inhibitor, aliskiren, was found to be as effective as the angiotensin receptor blocker (ARB), losartan, for regressing left ventricular hypertrophy (LVH) in hypertension patients in the ALLAY (ALiskiren Left ventricular Assessment. of hypertrophY) trial. But the combination of the two agents was no better than either one alone.
The study randomised 460 overweight hypertensive patients with evidence of LVH to one of three treatment arms: aliskiren 300 mg per day, losartan 100 mg per day, or aliskiren 300 mg per day plus losartan 100 mg per day, with all patients being treated to blood pressure targets. Treatment with the study drug was continued for 36 weeks.
The primary end point, left ventricular mass index (LVMI) as assessed by cardiovascular magnetic resonance imaging between baseline and 36 weeks, was reduced to a similar extent in all three groups. Although the reduction in LV mass was numerically greater in the combination arm, it failed to reach statistical significance. Aliskiren, either alone or in combination with losartan, was very well tolerated with no differences in adverse events between groups and a very low level of adverse events. There were no increases in hyperkalemia, hypotension or renal dysfunction in patients receiving aliskiren either alone or in combination, lead investigator, Dr Scott Solomon (Brigham and Women’s Hospital, Boston, US) reported.
MULTISTRATEGY: tirofiban and sirolimus stents beneficial in primary PCI
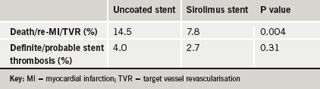
Sirolimus-eluting stents appeared safe and reduced target vessel revascularisation (TVR) in patients undergoing primary percutaneous coronary intervention (PCI) in the MULTISTRATEGY trial. The study also found that tirofiban, at its new higher dose, showed similar ST segment resolution to abciximab.
Dr Marco Valgimigli (University of Ferrara, Italy) explained that the use of drug-eluting stents in myocardial infarction (MI) patients is controversial because of the risk of stent thrombosis. In addition, the original dose of tirofiban was not as good as abciximab in PCI but a higher dose of tirofiban was now advocated. The MULTISTRATEGY trial was therefore conducted to look at these two issues.
The open-label 2 x 2 factorial study included 745 ST-elevation MI patients who were randomised to high-dose tirofiban vs. abciximab, and to sirolimus-eluting stent vs. bare metal stent. For the drug comparison, the main outcome measure was at least 50% ST-segment elevation resolution at 90 minutes post-intervention, which was seen in a similar amount of patients in the two groups (83.5% of abciximab patients vs. 85.3 of the tirofiban patients).
For the stent comparison, the primary end point was the rate of major adverse cardiac events, defined as the composite of death/MI/target-vessel revascularisation within eight months, which was significantly reduced in the sirolimus coated stent group. Stent thrombosis was similar between the two groups and the incidence of death/MI showed a trend towards a reduction in the sirolimus stent group, which said to be “reassuring”.
TRANSFER –AMI : transfer patients for PCI after lysis
A strategy of transferring myocardial infarction (MI) patients for percutaneous coronary intervention (PCI) within six hours of receiving thrombolysis is associated with significantly less ischaemic complications and no excess in bleeding in the TRANSFER-AMI (Trial of Routine Angioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction) study.
Lead investigator Dr Warren Cantor (Southlake Regional Health Centre, Ontario, Canada) suggested that this trial may have succeeded where others had failed because of the time window between lysis and PCI. He explained that if PCI is done too soon, the bleeding risk increases, but in this study, most patients received PCI about two-to-four hours after lysis, which seems to allow use of all the potent antithrombotic agents needed during PCI, without increasing risk. He also pointed out that this time interval was realistic for the real world.
In the study, around 1,000 patients were assigned to either thrombolysis (with rescue PCI for failed reperfusion and elective PCI encouraged for successfully reperfused patients after 24 hours) or a pharmacoinvasive strategy (transfer for PCI within six hours).
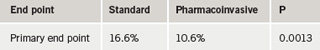
PCI was performed in 84% of patients in the pharmacoinvasive strategy compared with 62% of patients (after a mean of 27 hours) in the standard arm. The primary end point of the study, a composite of 30-day death, MI, heart failure, severe recurrent ischemia, or shock, was significantly lower in the transfer arm (table 1).
There was no difference in bleeding between the two groups.
ACC 2008: a primary care perspective
Dr Terry McCormack gives his views on some of the major trials presented at the ACC meeting and their implications for practice in the UK
This was an astonishing meeting, which was packed with new discovery and news. ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolaemia Enhances Atherosclerosis Regression) generated the most news and hullabaloo, but this was not about science so much as politics and finance. The trial itself tells us very little about ezetimibe, as most patients were pre-treated with statins and from the start had very little carotid intima to influence.
The New England Journal of Medicine article ‘Use of Ezetimibe in the United States and Canada’ by Jackevicius et al. (N Engl J Med 2008;358:1819–28) tells us the real story about this controversy. Ezetimibe accounted for 15.2% of the US market but only 3.4% of the Canadian market. The UK is very similar to the Canadian market. In December 2006 the monthly spend on Zetia® and Vytorin®, the two US ezetimibe brands, was US$ 261,479,000. Publication of the trial was delayed by more than a year, during which time the drugs bill exceeded US$ 4 billion. The American insurance companies and federal administrators are not happy.
Clearly, in the US, prescribers were replacing high-dose, newer statins with this unproven product. The message here is not to abandon ezetimibe, as it may well be proven to be effective by IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial), but rather to use it appropriately. This means using it after you have failed to achieve reasonable control with high-dose statins – in other words as a third-line drug, which is what most of us were already doing in the UK. By an amazing coincidence, the JUPITER (Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin) steering committee met on the same day and closed the trial because of a positive result favouring rosuvastatin.
Major hypertension studies report
The other amazing session was on the following day with the presentation of three major trials related to hypertension and prevention. ACCOMPLISH (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension) tells us that the calcium channel blocker amlodipine was significantly more effective than a thiazide diuretic in terms of preventing cardiovascular events in a high-risk population. But note this was a thiazide and not a thiazide-like diuretic. ALLHAT (Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial) used the thiazide-like diuretic chlorthalidone, which might explain the difference in outcomes.
HYVET (Hypertension in the Very Elderly Trial) proved a definite benefit in treating hypertension in people over 80 years for the first time. Some 80% of people in the treatment arm of this trial were taking both indapamide (also a thiazide-like diuretic) and perindropril. This may explain why we discovered not only that the treatment reduced strokes and all-cause mortality, but also that we reduced the relative risk of new onset heart failure by an incredible 64%.
The trial was independent in that it was funded by the British Heart Foundation and sponsored by Imperial College London. The drug costs were funded by Servier in the knowledge that the trial would close after the patent life of both drugs expired. It is important to recognise that this relates to fit people over 80 who do not have postural hypotension and where the target blood pressure is to below 150/90 mmHg. Adverse reactions were higher in the placebo group.
ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) was not a hypertension trial but shows that in terms of cardiovascular outcomes, the angiotensin receptor blocker (ARB) telmisartan is exactly as good as the angiotensin-coverting enzyme (ACE) inhibitor ramipril. It also tells us that combining an ACE inhibitor and ARB has no advantage in the non-heart failure patient and just increases side effects
Conflict of interest
TM has received research and educational grants from AstraZeneca, Boehringer-Ingelheim, MSD, Novartis, Schering-Plough, Servier and is an investigator and steering committee member for HYVET.
