Over the last few years, there has been a gradual increase in the use of intensive statin therapy, ostensibly atorvastatin 80 mg for high-risk individuals, such as those who suffered a myocardial infarction or underwent revascularisation. First-World economies, such as the UK, with a significant state contribution healthcare funding, had mounting anxiety because of the expanding indications and therefore cost of intensive statin therapy. The perceived relative expense of this strategy provoked a swift, often unilateral, withdrawal of such regimens by commissioners.
This drive towards generic prescribing for statins for all patients, with reduced efficacy, is against the published data from a large series of randomised-controlled trials and is counterintuitive. Funding organisations are being misinformed if they consider the swapping of effective treatment in this high-risk population is at all benign. This review seeks to demonstrate the strength of data and real-world experience suggesting that intensive statin therapies have real benefits for high-risk patients.
Introduction

There has been mounting controversy within a number of healthcare systems due to the expanding indications for statin therapy. Within the UK, there has been a gradual increase in the use of intensive statin therapy for high-risk individuals followed by a swift withdrawal of such regimens. This about face has been driven ostensibly by financial reasons, by primary care trusts (PCTs).
The preferred option by both PCTs and the Department of Health (DoH) is to use generic statins such as simvastatin, which are cheaper, but also less effective. Other agents such as atorvastatin and rosuvastatin are more expensive, but achieve greater reductions in cholesterol.
The move towards generic prescribing has been driven by the idea of more cost-effective prescribing, i.e. treating more people for less money. This is a laudable aim and one we should all aspire to. However, contemporaneously, cardiovascular risk management is being driven by care pathways and protocols, using clinical targets derived from different sources. These often use an up-titration strategy; increasing doses of the generic agent to reach a target. This in itself is a weakness – after all whose targets are we treating to, is the target low enough and how quickly should we get there?
I suspect that if these agents were equivalent in price to generic simvastatin, then this whole debate would become a non-issue. However, ignoring the cost disparity between agents for the moment, this conflict arises because the risk, and therefore the benefit, of all patient groups is not uniform. Treating a large number of patients with stable coronary artery disease (CAD) at low- to moderate- risk of cardiovascular events will certainly reduce the number of expensive hospital stays with cardiovascular events. However, the problem is that a smaller number of high-risk patients may also generate a significant number of adverse events and, unfortunately, post-acute coronary syndromes (post-ACS) patients fall into this category. In these patients the risk is front loaded and the modest immediate reduction of cholesterol does not provide an adequate reduction in cardiovascular risk; unfortunately cardiovascular events occur before the cholesterol has fallen adequately.
In this review, I hope to show the case for intensive lipid therapy as a logical therapeutic choice in high-risk cases such as post-myocardial infarction (post-MI). This strategy is not expensive and treats high-risk patients appropriately. The cost-effectiveness argument for stable patients can be developed in the future. However, in order to achieve
this it is necessary to answer a number of critical questions:
- Is intensive therapy more effective than moderate therapy?
- Do adverse events occur early?
- Can we take the time an up-titration
strategy requires? - Early intervention with statins reduces events, so how soon do we need to start a statin?
- Big doses are safe, so what is needed to get us over the prescribing inertia based on
issues of safety?
Not all statins are the same
Intensive statin therapy is accepted to include 80 mg simvastatin (just), atorvastatin 80 mg and rosuvastatin > 20 mg; although I would only really accept the latter two. The differences are well demonstrated in dose-ranging studies such as the Comparative Dose Efficacy Study of Atorvastatin versus Simvastatin, Pravastatin, Lovastatin and Fluvastatin in Patients with Hypercholesterolaemia (CURVES)1 and Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin (STELLAR).2
Figure 1, drawn from STELLAR, depicts the data available for those drugs in common usage in the UK: pravastatin 10–40 mg, simvastatin 10–80 mg, atorvastatin 10–80 mg and rosuvastatin 10–40 mg. It can be seen that only rosuvastatin 20–40 mg and atorvastatin 80 mg cross a mean percentage reduction in low-density lipoprotein cholesterol (LDL-C) of 50%.
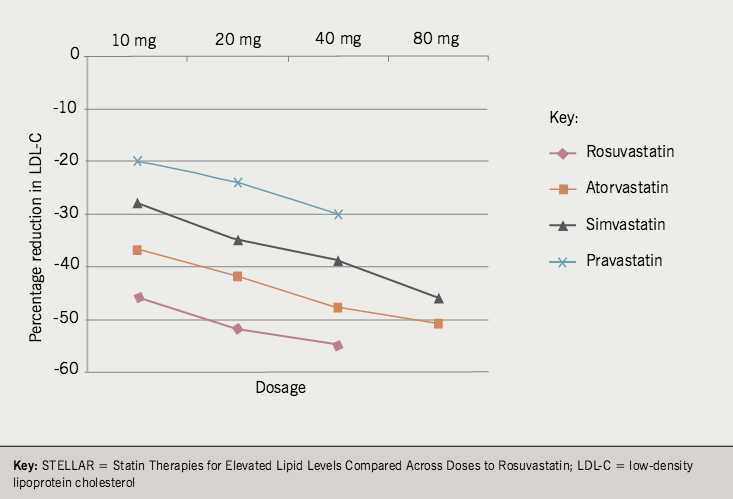
from the STELLAR trial
Most PCTs are pushing for generic simvastatin 20–40 mg, which produces a 39%2 or 41%1 reduction in LDL-C. However, the fraternity pushing for an intensive strategy are suggesting atorvastatin 80 mg, which produces a 51%2 or 54%1 reduction.
It is counterintuitive to accept a therapy that is 12–13% less effective in reducing cholesterol levels. The Cholesterol Treatment Trialists (CTT)3 collaboration, in their meta-analysis of 14 randomised controlled trials of more than 90,000 patients, clearly demonstrated that a 0.2 mmol/L reduction in LDL-C resulted in a reduction of 48 cardiovascular events for every 1,000 patients treated. This means that a 1 mmol/L reduction of LDL-C maintained for five years will reduce cardiovascular events by 23%. This was irrespective of the drug or the starting level of LDL-C. Mechanistic data from Reversal of Atherosclerosis with Aggressive Lipid Lowering Therapy (REVERSAL)4 and A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID)5 suggest that high-dose atorvastatin and rosuvastatin may actually arrest plaque progression. These intravascular ultrasound studies showed for the first time that intensive statin therapy and not moderate therapy could arrest or reverse plaque progression, an effect seen only with a > 50% reduction in LDL-C. This magnitude of LDL-C reduction is only seen with atorvastatin 80 mg or rosuvastatin > 20 mg.
The evidence to support intensive versus moderate statin therapy
The most robust data are drawn from four major trials: Aggrestat to Zocor (A to Z),6 Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT–TIMI 22)7 in ACS, Treating to New Targets (TNT)8 and Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL)9 in stable coronary heart disease (CHD).
PROVE-IT and A to Z
PROVE-IT7 compared atorvastatin 80 mg with pravastatin 40 mg, in 4,162 patients who had been hospitalised for recent ACS, to establish the non-inferiority of pravastatin compared with atorvastatin. This study was sponsored by Bristol Myers Squibb, the makers of pravastatin, rather than Pfizer. The moderate statin arm achieved a mean LDL-C level of 2.5 mmol/L compared with the intensive arm at 1.6 mmol/L. The primary end point was a composite of death from any cause, MI, unstable angina requiring rehospitalisation, revascularisation (> 30 days after randomisation), and stroke. A reduction in the primary event rate with intensive statin therapy compared with moderate therapy (26.3% vs. 22.4%, pravastatin vs. atorvastatin; p=0.005, 95% confidence interval [CI] 5–26%, number needed to treat [NNT] 26 over two years) was demonstrated.
A to Z6 was a double-blind randomised-controlled trial of ACS patients who received an initial 40 mg per day of simvastatin for one month followed by 80 mg per day thereafter (n=2,265) compared with ACS patients receiving placebo for four months followed by 20 mg per day of simvastatin (n=2,232). The primary outcome was major acute coronary events (cardiovascular death, non-fatal MI, re-admission for ACS, and stroke). Unfortunately only a trend towards supporting an intensive statin therapy was detected, which was not statistically significant as hoped (or expected).
Three hundred and forty-three patients (16.7%) in the placebo + simvastatin 20 mg group experienced the primary end point compared with 309 (14.4%) in the intensive statin arm (p=0.14, 95% CI 0.76–1.04). However, cardiovascular death occurred in 109 (5.4%) and 83 (4.1%) patients in the two groups (moderate dose versus intensive dose; hazard ratio [HR] 0.75, 95% CI 0.57–1.00, p=0.05) but no differences were observed in other individual components of the primary end point. Interestingly, no difference was evident during the first four months between the groups for the primary end point, but from four months onwards the primary end point was significantly reduced in the intensive statin group (HR 0.75, 95% CI 0.60–0.95, p=0.02).
It is important to investigate these findings more thoroughly as they appear to weaken the argument supporting intensive lipid-lowering strategies. A to Z investigated the impact of simvastatin in 4,500 patients at its termination, as planned, but was originally designed to recruit patients until 970 primary end point events occurred. These time points should have been contemporaneous but they were not. The primary end point events were slower to accumulate than anticipated, and unfortunately the Executive Committee were faced with a decision to terminate the study at 4,500 patients or to continue to 970 primary end point events. They chose the former, which left the study under powered and it consequently demonstrated a trend rather than a firmly positive outcome.
Wiviott’s analysis of PROVE-IT and A to Z10 considers the papers and amalgamates the data, but it is important to remember that this is a post hoc analysis of two different studies, although their excellent access to data made a thorough analysis possible. The main difference was in the early phase of both studies. In PROVE-IT, primary end point event reduction was seen early, within four months, and continued throughout the study. However, in A to Z, early benefit (< 4 months) was not seen, and the curves only separate later on (> 4 months). A to Z patients were recruited two days earlier (as part of the A phase), had more baseline risk factors, and were revascularised much less frequently. They also had a slower reduction in C-reactive protein level compared with atorvastatin patients in PROVE-IT. The lipid-lowering effects were similar between the two trials, and probably do not represent the reason for their differing outcomes. Beyond four months the trials are very similar in outcome. The conclusion reached is that the major reasons for A to Z’s apparent underperformance is the premature conclusion of the study and the frequency of revascularisation.
TNT and IDEAL
In stable CAD, the TNT8 and IDEAL9 studies both used atorvastatin 80 mg as the active comparator against atorvastatin 10 mg and simvastatin 20 mg, respectively. At first inspection, TNT demonstrated a result in favour of intensive lipid-lowering therapy; whereas IDEAL did not, which again demands further inspection.
TNT randomised 10,001 patients (median follow-up 4.9 years), with a primary outcome of CHD death, non-fatal MI, cardiac arrest or stroke. The study assessed lowering LDL-C levels below 2.6 mmol/L in patients with stable CHD, using either 10 mg or 80 mg of atorvastatin per day. The mean LDL-C levels were 2.0 mmol/L vs. 2.6 mmol/L for 80 mg vs. 10 mg atorvastatin, respectively. Primary events occurred in 434 patients (8.7%) vs. 548 patients (10.9%) for 80 mg vs. 10 mg of atorvastatin, respectively, representing an absolute reduction in the rate of major cardiovascular events of 2.2% and a 22% relative reduction in risk (HR 0.78; 95% CI 0.69–0.89, p<0.001). This strongly supports the intensive lipid-lowering strategy of 80 mg atorvastatin per day. The magnitude of lipid lowering afforded by treatment with 10 mg atorvastatin per day is similar to that seen with simvastatin 20–40 mg.
IDEAL looked at 8,888 people, with a history of acute MI (mean follow-up 4.8 years), randomised in an open-label fashion to receive a high dose of atorvastatin (80 mg per day; n=4,439), or usual-dose simvastatin (20 mg per day; n=4,449). The primary outcome measure was coronary death, confirmed non-fatal acute MI, or cardiac arrest with resuscitation. A primary outcome event occurred in 463 simvastatin patients (10.4%) and in 411 atorvastatin patients (9.3%) (HR 0.89; 95% CI 0.78–1.01, p=0.07), thus narrowly missing significance. Interestingly, non-fatal acute MI was significantly different between the groups and occurred in 7.2% vs. 6.0% for moderate vs. intensive strategy (HR 0.83; 95% CI 0.71–0.98; p=0.02). Similarly, major cardiovascular events occurred in 13.6% vs. 12% for moderate vs. intensive strategy (HR 0.87; 95% CI 0.77–0.98, p=0.02). The occurrence of any coronary event was reported in 23.8 vs. 20.2 (HR 0.84; 95% CI 0.76–0.91, p<0.001). Thus IDEAL failed to show significance for its primary major acute coronary events outcome measure, but significantly reduced other composite secondary end points and non-fatal acute MI.
IDEAL has been hijacked as a reason to abandon the intensive strategy by a number of organisations, but a more in depth look at the data does not support this view, rather it supports the opposite; the continued utility of the intensive strategy, both in post-ACS and stable CHD patient populations.
Meta-analysis seems to suggest a strong and cogent case for intensive statin therapy in patients with either recent ACS or stable angina. The magnitude of effect with intensive statin therapy is larger for ACS patients than seen in stable CHD. Indeed the more aggressively ACS patients are treated with early catheterisation and revascularisation strategies, the more they seem to benefit from intensive statin therapies.
Meta-analysis of the four studies
Cannon et al.11 reviewed the data from TNT, IDEAL, A to Z and PROVE IT. This meta-analysis investigated 27,548 patients (> 100,000 patient years) randomised to intensive versus moderate statin therapy. The combined analysis revealed a 16% odds reduction in coronary death or MI (p<0.00001) and a reduction of 16% in coronary death or any cardiovascular event (p<0.00001). A trend was also observed in cardiovascular mortality with an odds reduction of 12% (p=0.054). This is really as expected, as most studies of statins have indicated that their primary mode of action is to reduce the risk of recurrent ACS, ischaemia driven revascularisation and stroke. There was no difference in non-cardiovascular deaths 2.5% vs. 2.4% for intensive vs. moderate statin therapy (p=0.73).
More recently Afilalo et al.12 published a meta-analysis that included TNT, IDEAL, A to Z and PROVE-IT, but also included the Vascular Basis for the Treatment of Myocardial Ischemia Study13 and REVERSAL.4 Patients with a recent ACS benefited from a reduction in all-cause mortality from 4.6% to 3.5% with intensive statin therapy (95% CI 0.61–0.93), but this effect on all-cause mortality was not seen in stable CHD patients. Overall, intensive statin therapy significantly reduced major acute coronary events (odds ratio [OR] 3.72; 95% CI 2.10–6.57) and hospitalisations with heart failure (OR 3.72; 95% CI 2.10–6.57). This paper also reported a significant increase in transaminases over three times normal (OR 3.72; 95% CI 2.10–6.57) and a non-significant trend towards creatine kinase over 10 times normal and/or rhabdomyolysis (OR 1.96; 95% CI 0.50–7.63).
Early events and early treatment in ACS
Evidence abounds from studies of ACS treatments, where early event curves decay in an almost exponential way. Looking at the Efficacy and Safety of Subcutaneous Enoxaparin versus intravenous unfractionated heparin, in non-Q-wave Coronary Events (ESSENCE) trial14 as an example, which compared enoxaparin to unfractionated heparin in ACS. The event (death and MI) curves almost level off after 28 days, and in fact 50% of the events occur within the first week. This is typical of almost every ACS study. Events occur early, accrue rapidly and only begin to stabilise after four weeks.
This is important because if we accept the thesis that intensive statin therapy benefits this group, then it makes sense for the lipid-lowering effects to be achieved early – before or at least as the events are happening. The problem with some of the ACS statin trials has been that the median time before statins were initiated was too long. For example, in PROVE-IT the median time before statins were commenced was seven days. This generates two hypotheses: first that we should give statins as early as possible and second that we cannot reasonably take the time to up-titrate statins as is suggested in many PCT approved protocols. By the time patients achieve the modest proposed reductions in cholesterol, the vast majority of cardiovascular events have already occurred. Current PCT and DoH strategies probably miss the boat in ACS patients.
There are limited randomised data to support this, but the National Registry of Myocardial Infarction 4 (NRMI 4)15 demonstrates nicely that in-hospital death relates to the time of commencement of a statin. This registry draws data from 300,000 MIs in the USA and looked at patients in three groups; those on a pre-existing statin (17,000), those whose statin was started within 24 hours (22,000) and finally those whose statin was started after 24 hours (126,000). The in-hospital death rates are striking – those with pre-existing statin therapy had a 4% mortality, while those whose statin was commenced within 24 hours had a mortality of 5.3%. However, for those whose statin was commenced beyond 24 hours, the in-hospital mortality jumped to 15.4%. This strongly suggests that statin therapies should be started early in the course of a hospital stay in patients with a recent ACS.
Conclusion
In summary, I believe the evidence presents a cogent and robust argument for intensive statin therapy. This should include both ACS and stable CHD patients, although the magnitude of effect is greater in ACS patients.
Certainly in ACS patients, it is logical to hit LDL-C hard and to hit it early. Post-MI events are highest at the time of the index event. It is nonsensical to wait for these events to have occurred, with all their attendant risks, before commencing effective therapy. It is just this pre-emptive strategy that has driven the radically improved access to early in-patient angiography and revascularisation.
In Stoke on Trent, intensive statin therapy was the standard of care until it was withdrawn by the both the Hospital and Primary Care Trusts in late 2005. The introduction of generic simvastatin 40 mg was mandated. When the impact on patient events was studied,16 mortality was found to have risen from 5% to 17% and repeat cardiac admissions rose from 33 to 53, intensive to moderate statin therapy, respectively. Clearly, a switch to moderate statin therapy may not be safe or benign. Currently the local PCTs in North Staffordshire are considering revising the guideline to allow a one year use of intensive statin use post-MI.
Conflict of interest
RB has received honoraria for speaking and/or advisory board attendance from AstraZeneca, Daiichi-Sankyo, Novartis, Pfizer, Sanofi Aventis and Takeda. He has also received an educational grant from Pfizer and research nurse salary support from Sanofi Aventis.
Key messages
- Intensive therapy is more effective than moderate therapy in randomised trials and real life
- Adverse events occur early in myocardial infarction patients and we cannot take the time an up-titration strategy requires
- Early intervention with statins
reduces events - Intensive statin therapy is safe
References
- Jones P, Kafonek S, Laurora I et al. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 1998;81:
582–7. - Jones PH, Davidson MH, Stein EA et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92:
152–60. - Juliard JM, Himbert D, Cristofini P et al. A matched comparison of the combination of prehospital thrombolysis and standby rescue angioplasty with primary angioplasty. Am J Cardiol 1999;83:305–10.
- Nissen SE, Tuzcu EM, Schoenhagen P et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071–80.
- Nissen SE, Nicholls SJ, Sipahi I et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–65.
- de Lemos JA, Blazing MA, Wiviott SD et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292:1307–16.
- Cannon CP, Braunwald E, McCabe CH et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504.
- LaRosa JC, Grundy SM, Waters DD et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
- Pedersen TR, Faergeman O, Kastelein JJ et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437–45.
- Wiviott SD, de Lemos JA, Cannon CP et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials A to Z and PROVE IT-TIMI 22. Circulation 2006;113:1406–14.
- Cannon CP, Steinberg BA, Murphy SA et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006;48:438–45.
- Afilalo J, Majdan AA, Eisenberg MJ. Intensive statin therapy in acute coronary syndromes and stable coronary heart disease: a comparative meta-analysis of randomised controlled trials. Heart 2007;93:914–21.
- Stone PH, Lloyd-Jones DM, Kinlay S et al. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation 2005;111:
1747–55. - Cohen M, Demers C, Gurfinkel EP et al. Low-molecular-weight heparins in non-ST-segment elevation ischemia: the ESSENCE trial. Efficacy and Safety of Subcutaneous Enoxaparin versus intravenous unfractionated heparin, in non-Q-wave Coronary Events. Am J Cardiol 1998;82(5B):19L–24L.
- Fonarow GC, Wright RS, Spencer FA et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol 2005;96(5):611–16.
- Butler R, Wainwright J et al. Cholesterol lowering in patients with CHD and metabolic syndrome. Lancet 2007;369:27.
