This paper describes the experiences of developing a non-medical, non-catheter laboratory (cath lab) based implantable loop recorder (ILR) service. ILRs are small subcutaneous single-lead electrocardiogram (ECG) monitoring devices that are placed in a left pectoral pocket under local analgesia. Traditionally, devices have been implanted by medical staff in the cath lab. Each implant can take between 30 and 45 minutes depending on operator skill and patient anatomy. The development of this service has had several major patient and organisational benefits that include shorter waiting times, less cancellations and increased flexibility to implant ‘urgent’ devices in transient loss of consciousness (TLOC). The latter has reduced length of stay within our emergency assessment unit (EAU). Moreover, this service means that the department has been able to undertake more procedures in the cath lab. Data from 2013–14 suggest that an additional 32 × four-hour cath lab sessions were made available for alternative use. Adverse events (infection/erosion) are comparable with published data at less than 1%. To conclude, non-medical, non-cath lab based implantation is safe, cost-effective and has the potential to improve patient experience while increasing both cardiologist and cath lab capacity.
Introduction

This paper describes the development of a non-medical, non-catheter laboratory (cath lab) based implantable loop recorder (ILR) implantation service. The experiences of the team and results from prospective audit. When the service was first envisioned, the Lincolnshire Heart Centre had only one cath lab and was in the process of developing a primary percutaneous angioplasty (PPCI) service for ST-elevation myocardial infarction. As part of the business planning process we scrutinised what services were currently being undertaken where and by whom. We felt that with increasing inpatient demands and the need to generate cath lab capacity, that ILR implants should be moved from a consultant cardiologist led service within the cath lab to an alternative clinical environment. A treatment room on our medical day care unit (MDU) was identified and approved by the Trust’s infection prevention team. Initially, the service was undertaken by cardiology registrars who had received training from one of the consultant cardiologists. However, conflicting demands of day/night rotation, service delivery on the wards and clinical training meant that many sessions were cancelled. This led to long waiting times, patient complaints and poor patient satisfaction. As a result an alternative non-medical implantation service was developed.
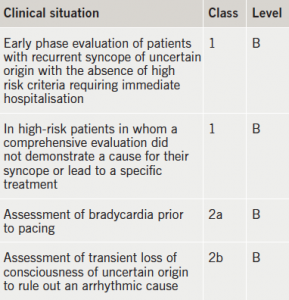
An ILR is a single-lead electrocardiogram (ECG) monitoring device that has until recently been implanted into a left pectoral pocket under local analgesia. Traditional devices take between 30 and 45 minutes to implant depending on operator skill and patient anatomy. More recently, an injectable device has become available that requires less surgical training than the previous devices. ILRs have advantages when compared with external rhythm monitoring devices as they can record over a longer period of time, have higher patient acceptability (not getting tangled in monitoring wires or having to replace electrodes) and record in total loss of consciousness where a ‘clutch’ type of monitor will not. Table 1 illustrates indications and evidence class for ILR in syncope.1
More recently, there is increasing evidence that a substantial proportion of cryptogenic strokes may be as a result of undiagnosed atrial fibrillation (AF) and the ILR may have an important role to play in ruling out AF as a cause of a patient’s events.2
In the UK, the National Institute for Health and Care Excellence (NICE) state “in patients that have total loss of consciousness with a probable cardiac cause, but have symptoms infrequently (less than once every two weeks) then an implantable loop recorder is the first line of investigation”.3
ILRs are more expensive than non-invasive monitoring and costs vary between £1,200 and £2,000 per device depending on the complexity of the device and institutional volume. Cost efficacy of ILRs in syncope has been scrutinised in several publications. The Centre for Research and Dissemination at the University of York undertook a probabilistic health economic assessment of ILRs (including implant, monitoring and explant costs) in the investigation of syncope compared with alternative methods of investigation and concluded that they were cost-effective.4
Methods
The Consultant Nurse in Cardiology (CN) who was already trained in consent, surgical skills and a non-medical prescriber was asked to develop a non-medical implant service, as it was envisioned this would result in a very short ‘start-up curve’ in getting the new service up and running. A period of supervised implantation under the guidance of a pacing consultant cardiologist was undertaken and competence was assessed using direct observation of procedural skills (DOPS). In order to deliver the highest quality clinical service with high patient flow during implant sessions, the CN was trained in ‘device mapping’, but routinely a clinical physiologist ‘mapped’ (identifying the optimum position for the device) and completed patient registration while CN implanted.
Within two months of the commencement of the new service, waiting lists had been cleared and patient-satisfaction audit scored high. As the team gained knowledge and skills the number of implants undertaken in a four-hour clinical session was increased from three to five (occasionally six). An unforeseen consequence of the new service was that demand increased by a factor of two, as referring clinicians realised the patients’ journey had improved.
The success of the service meant that a second operator was required to ensure continuity during annual leave and unplanned absence. One of the cardiology acute care practitioners (ACP) was identified and trained under the supervision of the nurse consultant. Two patients experienced anaphylaxis requiring treatment secondary to prophylactic pre-procedure antibiotics (teicoplanin used in penicillin allergic patients). Thus, a literature review was undertaken and no evidence was found that prophylactic antibiotic administration reduced pocket infection in ILR implantation, and, indeed, there was little evidence for their use in pacemaker implantation. Following discussion with microbiology, we stopped giving routine prophylactic antibiotics, and a prospective audit of n=100 patients was undertaken. No increase in pocket infection was noted. Routine cannulation was also discontinued. We also reviewed if devices can be implanted on warfarin provided the international normalised ratio (INR) <3 seconds to reduce patients thromboembolic risk in AF or those with prosthetic heart valves. Contemporary evidence suggested that pacemaker implantation could be undertaken on anticoagulated patients but no evidence was found for ILRs.5 As pacemaker implantation is more traumatic than ILR implantation, the pathway was revised to implant during continued anticoagulation. Diathermy is always available when implanting patients on warfarin/novel anticoagulation, but has never been required. No increase in bleeding/haematoma has been observed.
Physiology-led implantation
Historically, one of the Trust’s smaller hospitals had sent their patients to a neighbouring Trust for ILR implantation, as no local service was available. This meant a loss of revenue to the Trust, but more importantly long journey times for patients often undertaken on ‘public transport’. Many of our older patients found the journey arduous, particularly those on diuretic therapy or with impaired mobility. Hence, it was decided to develop a local service. A senior clinical physiologist (CP) expressed interest in developing this service and began training under the supervision of the CN. There were several challenges that needed to be overcome, namely that while a highly skilled physiologist, they had little training in surgical skills, consent or pharmacology when compared with a nurse practitioner. Legally, should a nurse or a clinical physiologist have a serious adverse event then they would be judged against the profession who had historically undertaken that role. Thus, to ensure consistent quality the clinical physiologist undertook the training in:
- Mental Capacity Act/consent
- European Resuscitation Council Advanced Life Support (ALS)
- Cannulation
- Intravenous drug administration
- Surgical skills
- ILR implantation/removal (30 supervised procedures).
It was necessary to amend the Trust’s medicines management policy to permit a clinical physiologist to administer medication, namely local anaesthetic. As clinical physiologists, who are a voluntary registered healthcare professional, are not legally permitted to administer medications under patient group directions (PGDs). However, they may administer against a named patient prescription order. Thus, options to facilitate clinical physiology administration are that the request form can be adapted to become a prescription, or the physiologist can approach an independent prescriber prior to the procedure and have a prescription order completed. Once trained, we amended the clinical physiologist job description to include ‘implantation/removal of loop recorders’ to ensure compliance with NHSLA (National Health Service Litigation Authority) requirements.
Injectable ILRs
Recently, an injectable ILR has become available. This device has several patient and organisational advantages, but also one major disadvantage that must be weighed against any advantages. Namely, it is more expensive than traditional devices. The new device is significantly smaller than the traditional devices and, thus, reduces implantation trauma and scarring (visible scarring on the chest is a very real consideration in younger female patients who are concerned about their body image, and we ensure that all patients are aware of the risk during the consent process). The device is injected into a left para-sternal pocket under local analgesic so requires less surgical training. It does not require surgical draping and is suited to clinic room implantation. Initially, when we started implanting this device we closed the wound with ‘steri-strips’ but later swapped to flexible surgical glue as it is less susceptible to movement. We also adopted a downward implantation technique rather than an upward technique, as this lessened device migration. Increased device cost is offset, as more devices can be implanted in a clinical session, thus, reducing staffing costs and administration costs. There is also a cost saving, as less equipment (draped/surgical packs) is needed to support implantation. The limiting factor on how many devices can be implanted in a session is now the time needed for patient education and physiology programing. We are able to implant eight devices in four hours. Ease of implantation has had an added patient and organisational bonus. Namely, we have been able to implant in ‘clinic rooms’ in the emergency department and emergency assessment unit. Thus, we have either avoided TLOC admissions or significantly reduced patient’s length of stay.
Results
Audit from 2012–14 (n=300) conducted on a number needed to treat (NNT) basis demonstrates an incidence of erosion/infection of <2%. If patients who have manipulated their device are removed then incidence of erosion/infection is <1%. Two patients suffered from a mild anaphylaxis reaction secondary to prophylactic antimicrobial therapy that is no longer routinely administered. No patients have had a major bleed/haematoma or major adverse event (on or off anticoagulation).
Based on the 2013–14 financial year (n=161) data, 32 × four-hour consultant cardiologist and cath lab sessions have been generated for other purposes. Potentially generating an additional 64 angioplasty/pacing spells with revenue in the region of £250k. It is more difficult to quantify the impact of 34 additional ‘patient activity’ consultant sessions. Length of stay for patients admitted with TLOC has been reduced and PROMS (patient recorded outcome measures) feedback has been excellent. Conversion from ILR to pacemaker was 12% in the first year but this is likely to increase year on year. Current devices have a battery life of three years. We are continuing to collect data in cryptogenic stroke.
Conclusion
We believe that we have demonstrated that non-medical, non-cath lab ILR implantation is both safe and effective. We have seen significant improvements in both the elective and emergent patient pathways and have reduced organisation expenditure while generating additional consultant and cath lab capacity.
Acknowledgment
The authors wish to thank Sheila Bampton, Cardiac Physiologist, who has supported their service over the last four years.
Conflict of interest
AR has undertaken a teaching session on non-medical ILR implantation for Medtronic. CM, JD, ARH, RA: none declared.
Key messages
- Implantation of implantable loop recorders (ILRs) outside of the catheter laboratory is safe and clinically as well as cost effective. It may improve the patient journey
- Nurses and senior physiologist can implant safely and efficiently
- New injectable ILRs reduce surgical trauma and scarring, and have high patient satisfaction levels.
References
1. Moya A, Sutton R, Ammirati F et al. Guidelines for the diagnosis and management of syncope (version 2009): The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J 2009;30:2631–71. http://dx.doi.org/10.1093/eurheartj/ehp298
2. Sanna T, Diener H-C, Passman RS et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. http://dx.doi.org/10.1056/NEJMoa1313600
3. National Institute for Health and Care Excellence.Transient loss of consciousness (‘blackouts’) management in adults and young people. CG109. London: NICE, 2010. Available from: http://www.nice.org.uk/guidance/cg109
4. Davis S, Westby M, Pitcher D, Petkar S. Implantable loop recorders are cost-effective when used to investigate transient loss of consciousness which is either suspected to be arrhythmic or remains unexplained. EP Europace 2012;14:402–09. http://dx.doi.org/10.1093/europace/eur343
5.ClinicalTrials.gov. Randomized study of the use of warfarin during pacemaker or ICD implantation in patients requiring long term anticoagulation. Identifier:NCT00721136. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00721136
