Understanding the mechanisms of AF lies at the heart of its treatment. AF occurs when structural and/or electrophysiological abnormalities alter atrial tissue to promote abnormal impulse formation and/or propagation (figure 1).3 Multiple clinical risk factors, electrocardiographic/echocardiographic features and biochemical markers are associated with an increased risk of AF (table 1), and, AF can be described in terms of the duration of episodes using a simplified scheme (table 2).3


The aim of treatment is to prevent stroke and alleviate symptoms.4 Drug therapies include anticoagulants to reduce the risk of stroke and anti-arrhythmics to restore/maintain the normal heart rhythm or slow the heart rate in patients who remain in AF.4 Non-pharmacological management options include electrical cardioversion, which may be used to ‘shock’ the heart back to its normal rhythm.4
Direct current (DC) cardioversion of AF was first reported in 1959,5 and this technique remains a reliable, safe and effective method to restore normal sinus rhythm.6 However, it has long been recognised that DC cardioversion is associated with a high risk of thromboembolic complications.7-9 It is now well established that anticoagulant therapy reduces the risk of stroke with the greatest benefit seen in patients at highest absolute risk.10-11 For several years, experts have recommended that patients who have had AF for more than two days should receive anticoagulation therapy for three weeks before elective cardioversion, and that anticoagulant should be continued until sinus rhythm has been maintained for four weeks.12 Unfortunately, anticoagulation therapy remains underused, particularly in the elderly, who doubtless have the most to gain from stroke prevention.10


of stroke risk and recommendation for
anticoagulation13,15
Risk stratification schemas are used to categorise patients into low, moderate and high stroke risk in order to initiate anticoagulant therapy in patients at highest risk.13 Historically, the most common stroke risk stratification system has been the CHADS2 score (Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, and prior Stroke or transient ischaemic attack [TIA]) within which a score of 0 categorises a patient as ‘low risk’, 1–2 as ‘moderate-to-intermediate risk’ and ≥3 as ‘high risk’.14 The most recent European Society of Cardiology (ESC) guidelines on AF recommend a new schema; CHA2DS2-VASc.15 The CHA2DS2-VASc scoring system places greater emphasis on the ‘major risk factors’ by allocating two points to each, with one point allocated for the presence of each of the other ‘clinically relevant non-major’ risk factors and anticoagulation thromboprophylaxis is initiated accordingly (table 3).13,15
Until recently, vitamin K antagonists (VKA), including warfarin, were the only oral anticoagulants recommended for antithrombotic therapy in patients at moderate-to-high risk of stroke, and, as such, warfarin has historically been the standard of care and foundation of thromboprophylaxis for stroke prevention in patients with AF.3,16 It is well established that the use of prophylactic warfarin in DC cardioversion significantly reduces the risk of ischaemic stroke and thromboembolism in patients with AF.11,17
DC cardioversion within the NHS
Under current National Institute for Health and Care Excellence (NICE) guidelines, rate control is the first-line treatment strategy for many adults with AF within the NHS.4 The guidelines recommend that DC cardioversion is used in AF patients whose symptoms continue after heart rate has been controlled (with beta blockers [first line], rate-limiting calcium-channel blockers, digoxin monotherapy or combination therapy) or for patients in whom a rate-control strategy has been unsuccessful.4
NICE guidelines advocate electrical (rather than pharmacological) cardioversion in patients with AF that has persisted for more than 48 hours.4 Also, as recommended by NICE, the CHA2DS2-VASc stroke risk score is routinely used within the NHS to assess stroke risk in patients with AF and in patients with AF for more than 48 hours, therapeutic anticoagulation is given for three to four weeks before cardioversion, and for at least four weeks afterwards, to minimise the risk of stroke.4 If the cardioversion is successful, anticoagulation may be stopped, however, patients at high risk of AF recurrence or increased risk of a stroke require continued treatment.4
In an emergency situation in patients with life-threatening instability caused by new-onset AF, NICE guidelines recommend that emergency electrical cardioversion should be performed without delay to achieve anticoagulation.4
Initiation and maintenance of warfarin in AF within the NHS
In the UK, anticoagulation is generally initiated within a secondary care setting, a dedicated hospital clinic or an outreach clinic in primary care.18 It takes 48–72 hours for the anticoagulant effect of warfarin to fully develop and warfarin should be taken at the same time each day.18
In direct contrast to patients with deep vein thrombosis or pulmonary embolism, rapid anticoagulation is not required in most patients with AF and a ‘slow-loading’ regimen is a safe and effective method of achieving therapeutic anticoagulation in the majority of patients within three to four weeks.18
Initiation at a dose of 1–2 mg per day is generally acceptable, with a lower starting dose in frail or elderly people and patients at high risk of bleeding.18 Warfarin treatment is usually long term for patients with AF, and the daily maintenance dose depends on the international normalised ratio (INR). A target INR of 2.5 is recommended for AF and, to achieve this, the daily maintenance dose of warfarin is usually 3–9 mg.18 However, significant variations in individual response due to a wide range of factors (age, genetics, concomitant drugs, diet, disease state) necessitate a daily dose between 1 mg and 15 mg in some patients.18
Cardioversion patients should achieve the target INR at least three weeks before cardioversion and four weeks after (if normal sinus rhythm is maintained).18
Non-vitamin K oral anticoagulants in cardioversion
Although effective in reducing the risk of thromboembolism, the limitations of warfarin, including a narrow therapeutic window, multiple drug and food interactions and the need for regular INR monitoring, present considerable challenges for its use in clinical practice.19-21 The challenges of maintaining warfarin within an appropriate therapeutic range combined with increased bleeding risk may contribute to issues with long-term treatment compliance.22
Four NOACs targeting either thrombin (dabigatran) or factor Xa (rivaroxaban▼, apixaban and edoxaban▼) have become available as alternative agents to warfarin.19,20,23–9 These NOACs offer the advantages of rapid onset of action, increased bioavailability, reduced half-life, fewer drug interactions and more predictable pharmacokinetic profiles compared with warfarin, thereby eliminating the need for routine coagulation monitoring (table 4).21,25–30
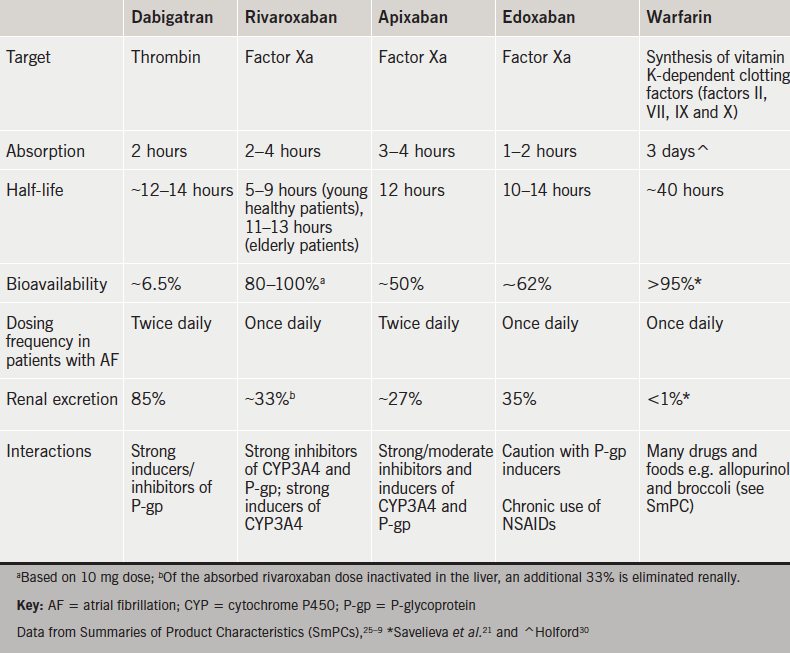
“NOACs represent an innovative treatment option and they are now generally being used as a default strategy for thromboprophylaxis in patients with AF,” reports Dr Craig Barr (Dudley Group NHS Trust). The use of NOACs for thromboprophylaxis is further supported by Dr Shaumik Adhya (Medway Foundation Trust and Honorary Consultant Cardiologist at Guy’s and St Thomas’ NHS Foundation Trust) who confirms, “Clinicians are confident in the use of NOACs and favour their flexibility.”
Large randomised-clinical trials (RCTs), as well as post hoc analysis, have demonstrated comparable efficacy and safety profiles compared with warfarin in non-valvular AF patients undergoing cardioversion (table 5).31–6
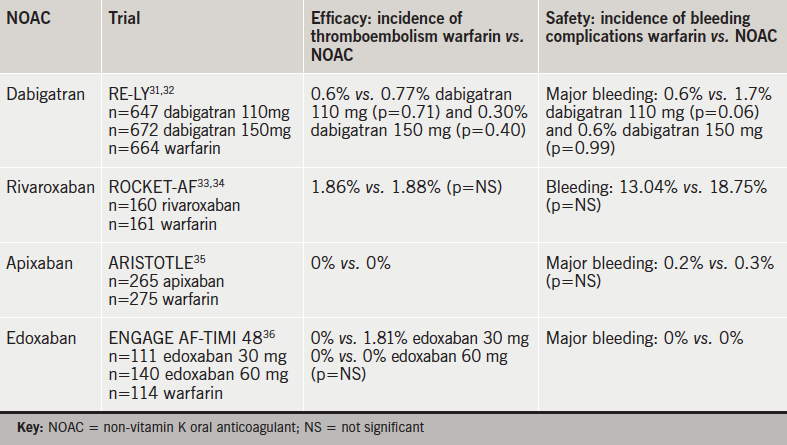
Although the RE-LY (Randomized Evaluation of Long-term anticoagulation therapY) trial was not powered to detect differences in the absolute risk of embolism in patients undergoing cardioversion, results indicate comparable efficacy and safety profile of dabigatran versus warfarin and suggest that dabigatran may be a realistic alternative to warfarin in these patients.31,32
A post-hoc analysis of AF patients who underwent cardioversion in the ARISTOTLE study (Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation) demonstrated comparable data for apixaban versus warfarin, as no stroke or systemic embolisms occurred in either warfarin or apixaban patients during a 30-day post-cardioversion follow-up.35
A post hoc analysis of the ENGAGE AF-TIMI 48 study concluded that thromboembolic and major bleeding events post cardioversion were infrequent and similar with edoxaban and warfarin.36,37
Comparable efficacy and safety profiles of rivaroxaban in patients undergoing cardioversion was demonstrated by post-hoc analysis of the ROCKET-AF (Rivaroxaban Once-daily oral direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial.33,34 Results show a similar rate of stroke/systemic embolism with no significant difference in the incidence of major bleeding, but increased gastrointestinal bleeding, suggesting that rivaroxaban may be used as an alternative to warfarin in these patients.33,34
There is currently limited evidence supporting the use of NOACs in elective cardioversion (elective cardioversion was an exclusion criteria in the ROCKET-AF trial).33 The X-VeRT trial was designed to investigate the efficacy and safety profiles of rivaroxaban versus VKA in non-valvular AF patients undergoing elective cardioversion.38,39 The trial included 1,504 adult patients with haemodynamically stable non-valvular AF for more than 48 hours (or unknown duration) scheduled for elective cardioversion. Patients were randomised in a 2:1 ratio to anticoagulation with rivaroxaban (20 mg once daily [od]; 15 mg od in patients with renal impairment) or standard VKA therapy using one of two cardioversion strategies (early or delayed) and all patients received study treatment for six weeks post-cardioversion (figure 2). Early cardioversion involved rivaroxaban or standard VKA therapy one to five days before cardioversion, while delayed cardioversion involved rivaroxaban or standard VKA therapy 21 to 56 days before cardioversion.39

Results from X-VeRT demonstrate that rivaroxaban is comparable in efficacy and safety profiles to VKA therapy in non-valvular AF patients undergoing elective cardioversion. Overall, thromboembolism events occurred in 0.51% of rivaroxaban patients versus 1.02% of VKA patients (risk ratio 0.50; 95% confidence interval [CI] 0.15–1.73). In the rivaroxaban group, 0.71% of patients experienced thromboembolism events following early cardioversion and 0.24% following delayed cardioversion. In the VKA group, 1.08% of patients experienced thromboembolism events following early cardioversion and 0.93% following delayed cardioversion. Rivaroxaban was also associated with a significantly shorter time to cardioversion compared with VKAs (p<0.001) and risk of major bleeding was low and similar to that observed with VKA treatment (0.6% vs. 0.8% in the VKA group [risk ratio 0.76; 95% CI 0.21–2.67]). The results of the X-VeRT trial are aligned with the conclusions drawn from the ROCKET-AF study; rivaroxaban has the safety profile and is an effective alternative to warfarin in patients requiring anticoagulation therapy prior to, and following, cardioversion and may facilitate more rapid cardioversion.33,34,39
In January 2015, the European label for rivaroxaban was updated based on the findings from the X-VeRT study. Rivaroxaban is currently the only NOAC for initiation or continuation of anticoagulation in patients with non-valvular AF to demonstrate specific data for elective cardioversion within its SmPC.26 By comparison, the current data available support the use of dabigatran, apixaban and edoxaban only in patients to be continued on these agents if scheduled for cardioversion; the licences do not allow physicians to switch patients currently on warfarin to any of these NOACs.25,27
According to the current ESC guidelines and the joint recommendations by the European Society of Cardiology (ESC), the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA) and the Heart Rhythm Society (HRS), NOACs should be considered in most patients with non-valvular AF as an alternative to an adjusted dose VKA,3,16 and specific recommendations at a European level are described in table 5.
Due to the heterogeneity of key RCTs and lack of head-to-head data, it is difficult for the guidelines to recommend one NOAC over the other. However, several practical considerations can be helpful in the selection of an appropriate agent for individual patients (figure 3).20

UK experience of NOACs in cardioversion
All four currently licensed NOACs have been approved by NICE as options for the prevention of stroke and systemic embolism in patients with non-valvular AF4 and are recommendations by the ESC for prevention of stroke and systemic embolism in patients with non-valvular AF (table 6).20

Kent
Kent’s regional experience of the use of NOACs in cardioversion to date has been very positive, reports Dr Shaumik Adhya (Medway Foundation Trust). Healthcare professionals (HCPs) from centres across the region have offered positive ‘real-world’ feedback on the initiation of patients on NOACs, particularly rivaroxaban, in their practices. Overall, clinicians are confident in their use of NOACs; they favour the flexibility and report that NOACs make surgical procedures more convenient.
Dr Adhya notes that the centres were in agreement that the need to counsel patients on the importance of adherence, and the possible consequences of non-adherence, remain the priority when initiating or continuing NOAC therapy prior to cardioversion. One concern associated with NOACs in this setting relates to compliance; many centres insist on signed pro-forma patient consent forms, clearly stating the responsibility of the patient in adhering to their treatment with NOACs prior to the procedure.
Dr Adhya reports that rivaroxaban remains the most frequently prescribed NOAC in the region, and the benefits, thus far, include a reduction in clinician consultation times and a reduction in cardioversion waiting lists due to a decrease in the number of patients previously unable to undergo scheduled cardioversion due to sub-therapeutic INR levels.
The Midlands
With three years and nearly 2,000 patient-years of experience of routine NOAC use at Russells Hall Hospital in Dudley, HCPs report positive clinical results and encouraging patient feedback. Dr Craig Barr (Dudley Group NHS Trust) highlighted how NOACs are now generally being used as a default strategy in patients with non-valvular AF to prevent stroke. He highlighted how rivaroxaban has generally been chosen as the preferred NOAC for a variety of reasons, including its once-daily dosing, its multiple indications and few drug interactions. This strategy, he said, had led to highly effective predictable anticoagulation, and no need for dietary restrictions or routine coagulation monitoring in patients.
Experience of using NOACs with patients undergoing cardioversion has been encouraging, reports Dr Barr. Ease of use and rapid onset of action are beneficial from both a patient and HCP perspective, with patients reporting an overall more favourable experience compared with warfarin. Patients are confident that the benefits outweigh the disadvantages and are reassured by the lower risk of bleeding.
HCPs in Dudley report clear-cut clinical advantages of NOACs including comparable efficacy to warfarin without the disadvantages and inconvenience associated with INR testing and drug/food interactions. The routine use of NOACs in the Midlands has resulted in timelier cardioversions (which may impact upon a greater long-term maintenance of sinus rhythm), very few cancellations and a shorter waiting time for cardioversion procedures.
HCPs report that, although NOAC compliance is an important aspect that is discussed with all patients in nurse-led clinics that are run on a weekly basis, after more than three years of experience, adherence is not a significant issue and patient consent is considered unnecessary and not routinely sought.
London
After more than two years of routine use, arrhythmia nurse Evaun Teoh (St George’s University Hospitals NHS Foundation Trust) reports “HCPs are extremely comfortable using all three of the currently available NOACs for cardioversion and stroke prevention.”
She further reports that, although clinician preference is a factor, choice of specific NOAC is primarily guided by specific protocols that take into consideration renal clearance and a patient’s prospects of compliance; younger patients are generally prescribed a daily NOAC, while older patients are considered more compliant (unless evidence of memory impairment) and are prescribed a twice-daily NOAC.
She feels that clinicians are reassured by the immediacy of effects, and patient feedback has been overwhelmingly positive, with patients reporting benefits in terms of ease of use and feelings of independence. Although some patients report rashes or gastrointestinal side effects, these usually occur following a change in dose and are generally mild.
St George’s have adopted a comprehensive specialist nurse-led patient-counselling programme to ensure NOAC compliance. The vital importance of compliance to ensure continuous efficacy is explained to the patient before a NOAC is initiated. The specialist nurse remains a long-term point of contact and various strategies, such as smart phone alerts, are in place to help patients remain compliant. The concept of ‘degrees of bleeding’ is also explained and all patients are advised to carry a patient alert card in case of internal/severe bleeding.
Setting up a pathway for a NOAC in cardioversion
The management of AF is complex and costly, and integrated care pathways (ICPs) are required to ensure optimal management and outcomes.
ICPs are a vital mechanism to ensure consistency and quality in healthcare; the overall purpose of ICPs is to improve outcome by providing a mechanism to coordinate care and to reduce fragmentation and ultimately cost.40
According to continuous quality improvement principles, the primary goal of clinical pathways for NOACs in cardioversion should be to improve clinical efficacy and efficiency within the healthcare system. Pathways are an evidence-based response to specific problems and care needs.
Although there are many potentially ‘ideal’ pathways, developing ICPs as part of an improvement project should follow the outline of ‘Improving the patient pathway’ published by the NHS Institute for Innovation and Improvement,41 and it has been documented that pre-cardioversion ‘work-up’ may be improved by installing clinical pathways led by nurse practitioners or physician assistants.42
Figure 4 describes a potential pathway for the use of NOACs in cardioversion as used in various hospitals in Kent.

Real-life experience: typical patient pathways on a NOAC versus warfarin
The ‘real-life’ experiences of two cardiologists and a nurse specialist from three regional centres have provided important insights into ‘typical’ patient pathways in the ‘real-world’. These are described in this section.
The experts note that a typical patient will present in primary care and, as a result, anticoagulation will be initiated by the GP. However, in reality a significant proportion of patients (around 40%) present as emergency or coronary care cases.
It is clear that the use of warfarin versus NOACs in a ‘real-life’ primary care setting is highly variable, and often based on historical anticoagulant use by the GP, lack of knowledge and potentially a lack of confidence in NOACs. As a consequence, warfarin is often initiated as the anticoagulant within the primary care setting. However, patients with a history of labile INRs, frequent travellers or patients taking antibiotics are often switched to NOACs for convenience.
In contrast, demonstrated efficacy and the advantages of simplicity of use have meant that NOACs are increasingly accepted and regularly initiated in the secondary care setting as the ‘default’ procedure. The experts interviewed report that HCPs in secondary care routinely initiate anticoagulation with NOACs (primarily rivaroxaban) no more than 21 days after presentation.
The experts note that potential for compliance may be a ‘real-life’ issue affecting the decision to initiate NOAC versus warfarin and HCPs report that warfarin may be a preferable option in potentially non-compliant patients. Conversely, diet, alcohol and use of over-the-counter medications impact the efficacy of warfarin, and the experts report these may be ‘real-life’ factors affecting choice of anticoagulant.
Once initiated, patients remain on anticoagulation for four weeks, after which, they are assessed to see if longer-term anticoagulation is required. This is subsequently initiated in the vast majority of cases, with many patients requiring life-long NOAC treatment. After a year of monitoring in secondary care, patients are usually then followed-up by their GP.
The experts reported that patients experience less challenging side effects with NOACs and although INR testing has become more easily accessible (self-testing/pharmacy testing now available), most patients prefer the convenience and flexibility offered by NOACs.
Electrical cardioversion of non-valvular AF with the NOACs: a single-centre UK-based registry experience
This registry-based study involved analysis of all consecutive patients undergoing electrical cardioversion between January 2013 and March 2014.43 A total of 229 procedures were performed during this period; 122 were anticoagulated with a NOAC (rivaroxaban 120, dabigatran 2, and mean age 63 ± 12, female 31%) and 107 with warfarin (mean age 67 ± 10, female 41%).
Overall, the results from the study revealed:43
- No major bleeding episodes, stroke, thromboembolic events or death occurred in either group
- Significantly fewer cancellations in the rivaroxaban group (1 patient [0.8%] due to dose omission vs. 11 patients [10.3%] in the warfarin group due to subtherapeutic INRs on the day of the procedure, p=0.004)
- Shorter mean procedural waiting time in the NOAC group in comparison with warfarin group (mean ± standard deviation [SD] = 67 ± 49 vs. 95 ± 66 days, p=0.006)
- A trend towards greater procedural success in the NOAC group, 94% (115/122), compared with warfarin, 79% (85/107, p=0.001).
This single-centre experience of cardioversion along with the NOACs demonstrates that the procedure is effective and has a good safety profile. Cardioversion is performed in a more timely fashion, with very few cancellations and shorter waiting time, which may impact upon a greater long-term maintenance of sinus rhythm.43
Looking to the future
The NOACs are set to provide a realistic therapeutic alternative to VKAs in non-valvular AF patients undergoing cardioversion, providing a therapeutic option that reduces clinic waiting times, reduces time spent in hospital for patients and minimises rescheduling of procedures due to subtherapeutic INRs.
The use of NOACs in cardioversion should involve an approach based on the published data, patient comorbidities and procedural factors,20 and policies need to be developed for integration of NOACs into local care pathways.44 There is a requirement for alignment of treatment protocols across primary and secondary care and a NOAC should be considered for newly diagnosed patients and as a ‘switch’ in certain VKA patients.44
In patients scheduled for cardioversion, a NOAC should be the preferred option to enable early cardioversion, which can occur approximately three weeks after initiation of the NOAC. Extra counselling should be performed to ensure that the patient fully understands the necessity for compliance, as non-compliance would leave the patient unprotected against thromboembolism. A proforma is signed by the patient and counsellor, with emphasis on drug compliance pre- and post-cardioversion.44
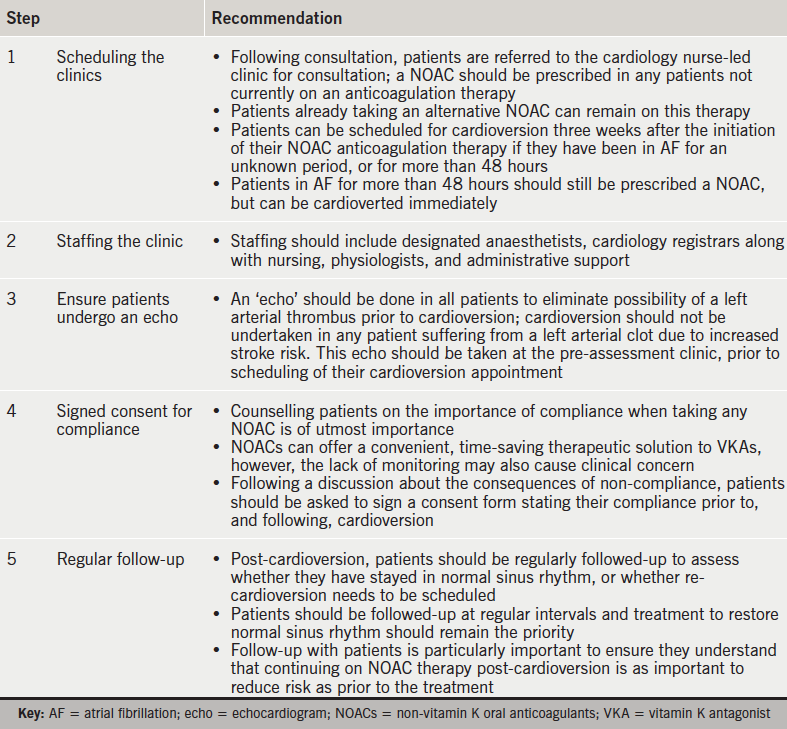
Practical steps to ensure correct clinic set-up are outlined in table 7.
Summary
The high risk of stroke associated with DC cardioversion can be reduced by oral anticoagulation, and NOACs have demonstrated comparable efficacy and safety profiles with warfarin for thromboprophylaxis in AF patients undergoing cardioversion.
It is important that local arrangements for use of antithrombotic therapies in non-valvular AF should be reviewed and policies developed for integration of NOACs into the care pathways.44 Primary care prescribing of NOACs needs local leadership, as GPs should not be expected to be experts in the area of anticoagulation for AF, and as the AF ‘epidemic’ continues to increase, local anticoagulant ‘champions’ will be required to take the lead.44
References
1. Davis RC, Hobbs FD, Kenkre JE et al. Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace 2012;14:1553–9. http://dx.doi.org/10.1093/europace/eus087
2. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17–40. http://dx.doi.org/10.1016/j.mcna.2007.09.002
3. January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. http://dx.doi.org/10.1161/CIR.0000000000000040
4. National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. CG180. London: NICE, June 2014. Available from: https://www.nice.org.uk/guidance/cg180 [accessed July 2015].
5. Vishnevskii AA, Tsukerman BM, Smelovskii SI. Control of fibrillating arrhythmia by the method of electrical defibrillation of the atrium. Klin Med (Mosk) 1959;37:26–9.
6. Cakulev I, Efimov IR, Waldo AL. Cardioversion: past, present, and future. Circulation 2009;120:1623–32. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.865535
7. Lown B. Electrical reversion of cardiac arrhythmias. Br Heart J 1967;29:469–89. http://dx.doi.org/10.1136/hrt.29.4.469
8. Resnekov L, McDonald L. Complications in 220 patients with cardiac dysrhythmias treated by phased direct current shock, and indications for electroconversion. Br Heart J 1967;29:926–36. http://dx.doi.org/10.1136/hrt.29.6.926
9. McCarthy C, Varghese PJ, Barritt DW. Prognosis of atrial arrhythmias treated by electrical counter shock therapy. A three-year follow-up. Br Heart J 1969;31:496–500. http://dx.doi.org/10.1136/hrt.31.4.496
10. Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol 2007;6:981–93. http://dx.doi.org/10.1016/S1474-4422(07)70264-8
11. Gallagher MM, Hennessy BJ, Edvardsson N et al. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol 2002;40:926–33. http://dx.doi.org/10.1016/S0735-1097(02)02052-1
12. Singer DE, Albers GW, Dalen JE et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(suppl 6):546S–592S.
13. Lip GY. Stroke in atrial fibrillation: epidemiology and thromboprophylaxis. J Thromb Haemost 2011;9(suppl 1):344–51. http://dx.doi.org/10.1111/j.1538-7836.2011.04302.x
14. Gage BF, Waterman AD, Shannon W et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–70. http://dx.doi.org/10.1001/jama.285.22.2864
15. Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. http://dx.doi.org/10.1093/eurheartj/ehq278
16. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. http://dx.doi.org/10.1093/eurheartj/ehs253
17. Arnold AZ, Mick MJ, Mazurek RP et al. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol 1992;19:851–5. http://dx.doi.org/10.1016/0735-1097(92)90530-Z
18. National Institute for Health and Care Excellence. NICE Clinical Knowledge Summaries. Anticoagulation oral. London: NICE, February 2015. Available from: http://cks.nice.org.uk/anticoagulation-oral#!changes [accessed July 2015].
19. Mann A, Ruskin J, Heist EK. Cardioversion and catheter ablation of atrial fibrillation using novel oral anticoagulants. The Journal of Innovations in Cardiac Rhythm Management 2014;5:1792–9.
20. Maan A, Heist EK, Ruskin JN, Mansour M. Practical issues in the management of novel oral anticoagulants – cardioversion and ablation. J Thorac Dis 2015;7:115–31. http://dx.doi.org/10.3978/j.issn.2072-1439.2014.11.35
21. Savelieva I, Camm AJ. Practical considerations for using novel oral anticoagulants in patients with atrial fibrillation. Clin Cardiol 2014;37:32–47. http://dx.doi.org/10.1002/clc.22204
22. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer Adherence 2010;4:51–60.
23. Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program 2013;2013(1):464–70. http://dx.doi.org/10.1182/asheducation-2013.1.464
24. Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract 2010;64:956–67. http://dx.doi.org/10.1111/j.1742-1241.2009.02286.x
25. Pradaxa. Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf [accessed July 2015].
26. Xarelto. Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf [accessed July 2015].
27. Eliquis. Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf [accessed July 2015].
28. Lixiana. Summary of Product Characteristics. Available from: http://www.medicines.org.uk/emc/medicine/30506 (accessed July 2016)
29. Warfarin. Summary of Product Characteristics. Available from: http://www.medicines.org.uk/emc/medicine/23933 (accessed July 2016)
30. Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet 1986;11:483–504. http://dx.doi.org/10.2165/00003088-198611060-00005
31. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. http://dx.doi.org/10.1056/NEJMoa0905561
32. Nagarakanti R, Ezekowitz MD, Oldgren J et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011;123:131–6. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.977546
33. Patel MR, Hellkamp AS, Lokhnygina Y et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). Am Coll Cardiol 2013;61:651–8. http://dx.doi.org/10.1016/j.jacc.2012.09.057
34. Piccini JP, Stevens SR, Lokhnygina Y et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol 2013;61:1998–2006. http://dx.doi.org/10.1016/j.jacc.2013.02.025
35. Flaker G, Lopes RD, Al-Khatib SM et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol 2014;63:1082–7. http://dx.doi.org/10.1016/j.jacc.2013.09.062
36. Plitt A, Ezekowitz MD, De Caterina R, et al. on behalf of the ENGAGE AF-TIMI 48 Investigators. Cardioversion of atrial fibrillation in ENGAGE AF-TIMI 48. Clin Cardiol 2016;39:345–6. http://dx.doi.org/10.1002/clc.22537
37. Giugliano RP, Ruff CT, Braunwald E, et al. for the ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. http://dx.doi.org/10.1056/NEJMoa1310907
38. Ezekowitz MD, Spahr J, Ghosh P, Corelli K. Stroke prevention in atrial fibrillation: established oral anticoagulants versus novel anticoagulants – translating clinical trial data into practice. J Interv Card Electrophysiol 2014;40:261–8. http://dx.doi.org/10.1007/s10840-014-9893-z
39. Cappato R, Ezekowitz MD, Klein AL et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J 2014;35:3346–55. http://dx.doi.org/10.1093/eurheartj/ehu367
40. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care 2003;15:509–21. http://dx.doi.org/10.1093/intqhc/mzg057
41. NHS Institute for Innovation and Improvement. Improving patient pathways. Available from: http://www.institute.nhs.uk/care_outside_hospital/care/care_outside_hospital.html [accessed July 2015].
42. Deuling JH, Vermeulen RP, Smit MD et al. Planning and monitoring of patients for electrical cardioversion for atrial fibrillation. Neth Heart J 2012;20:148–54. http://dx.doi.org/10.1007/s12471-011-0208-z
43. Arujuna A, Ooues G, Abbas A et al. Electrical cardioversion of atrial fibrillation with the novel oral anticoagulants: a single centre UK-based registry experience. Europace 2014;16(suppl 3):iii14. http://dx.doi.org/10.1093/europace/euu239.11
44. NICE Implementation Collaborative Consensus. Supporting local implementation of NICE guidance on use of the novel (non-Vitamin K antagonist) oral anticoagulants in non-valvular atrial fibrillation. London: NICE, June 2014. Available from: http://www.nice.org.uk/guidance/cg180/resources/nic-consensus-statement-on-the-use-of-noacs-243733501

