Endomyocardial biopsy (EMB) has been long established as a diagnostic tool in myocardial disease. EMB surveillance for rejection of cardiac allografts continues to be routinely performed. However, the use of EMB beyond transplant monitoring is controversial. In recent years, the procedure has fallen out of favour. This is most likely due to the growing capabilities of non-invasive imaging modalities and the questionable impact of EMB findings on treatment.
This article aims to examine current practice of EMB in England, discuss the utility of EMB in myocardial diseases and compare prominent society guidelines from recent years. Information gained from freedom of information requests shows just 18% of NHS trusts reported performing EMB, and only 46% referred to other centres for EMB in England in 2014–2015. Despite the limitations of EMB, it remains the only procedure capable of obtaining a histological diagnosis of cardiac disease.
Introduction
Endomyocardial biopsy (EMB) was first pioneered in 1962 in Japan.1 Since then EMB has undergone significant advances in procedural equipment and tissue analysis.2,3 Using a combination of histological, immunohistochemical and viral analysis, a variety of myocardial disorders can be detected.
The procedure involves taking four or more biopsy samples from the right intraventricular septum using flexible bioptomes, under fluoroscopic or echocardiographic guidance.4 Routine access is typically via a sheath inserted into the right internal jugular or femoral vein. Left ventricular biopsy can also be performed via the femoral arteries with separate left ventricular bioptomes, but this is less commonly performed.5
A frequently cited concern regarding EMB is safety of this invasive test. However, the procedure carries a relatively low complication rate of less than 1% in experienced centres.6,7 Significant potential complications still include right ventricular wall rupture, conduction block, arrhythmia, pneumothorax, tricuspid regurgitation and pulmonary embolisation.8 Procedural-related death is rare in most recent series, but has been reported previously.6
Currently, there are no available data regarding provision and application of EMB in the National Health Service (NHS).
Materials and methods
The study aimed to define availability of EMB and current practice of use of EMB in myocarditis disorders in NHS hospitals in England using reporting via freedom of information (FOI). FOI requests were served to all 137 trusts providing acute cardiology services in England. All trusts were asked four main questions:
- Do any hospitals in the trust perform endomyocardial biopsy?
- If so, how many were performed in the 2014–2015 financial year?
- How many were performed for investigation of myocarditis?
- If not, does the trust refer patients to other centres for endomyocardial biopsy?

Results
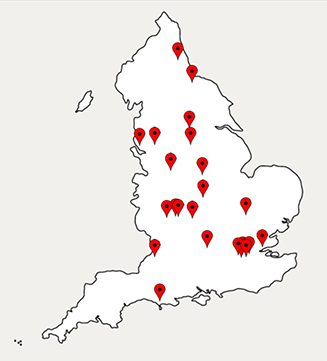
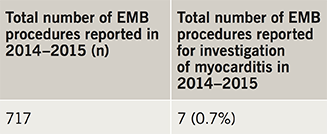
The response rate was 97% (133/137 trusts). Reporting from these trusts showed that only 24 trusts (18%) performed EMB in 2014–2015 (table 1, figure 1). All biopsy-performing trusts offered tertiary level cardiology services. A total of 717 biopsy procedures were reported by all trusts within this period and only seven (0.9% of all biopsies) were reportedly performed for the diagnosis of myocarditis in this time period (table 2). Referring patients to a tertiary centre for EMB was reported by 61 trusts (46%), but many noted this was rare. Reporting of EMB procedure numbers was variable and may be subject to reporting low-level inaccuracies but appears to largely represent current practice. While this clearly outlines practice in England, no data from Scotland, Wales and Northern Ireland are currently available.
Discussion
EMB in transplant monitoring
Routine EMB surveillance for rejection of cardiac allografts is well established within transplant centres. Biopsy provides histological monitoring for cell-mediated or antibody-mediated rejection and should be performed periodically within the first year post-transplant.9 Necessity for further monitoring after a year depends upon an individual’s risk of rejection. Continued EMB surveillance after six months in asymptomatic patients has been scrutinised and is likely to be of limited benefit.10,11 Cardiac magnetic resonance (CMR) and gene expression profiling have been shown to be useful in assessment for rejection.12,13 However, while these may act as good pre-test predictors of rejection, EMB remains the gold-standard diagnostic test to assess rejection.
EMB in myocardial diseases
The role of EMB beyond transplant monitoring has been more contentious. Many feel EMB has a role in the diagnosis of cardiomyopathy and myocarditis.8,14 The ability of EMB to detect viral infection and establish the histological pattern of myocarditis has potential benefit for patients who may respond to either antiviral or immunosuppressive therapies.
Viral myocarditis
Viral myocarditis is the most common aetiology of myocarditis. The viral pathogens most frequently implicated in myocarditis include coxsackie A and B, cytomegalovirus, Epstein–Barr and human herpes virus 6.15 Advances in the use of polymerase chain reactions (PCR) and viral load measurement techniques have significantly improved isolation of viral DNA in myocardial tissue. Presence of persistent enteroviral genomes in biopsy samples has been associated with worsening functional status and mortality.16 There has been a particular interest in the association between persistent viral myocarditis and subsequent dilated cardiomyopathy.17 Using these viral detection techniques there is hope that appropriate anti-viral therapies may be used to treat patients with persistent viral infection, halting any progression to cardiomyopathy.
However, specific treatments for viral myocarditis have yet to be established. While animal studies have previously suggested a protective effect of interferon in mice, there are few clinical trials in humans.18 A small, single-centre study of 22 patients given interferon-beta therapy demonstrated statistically significant improvements in left ventricular function and mortality over 10 years.19 No further larger multi-centre studies have, as of yet, validated these findings. Systematic reviews of corticosteroids and immunoglobulin have shown no reduction in mortality.20,21 There is hope that with further research and larger clinical trials therapeutic options may be found for patients with persistent infection.
Inflammatory myocarditis
Distinguishing histological patterns of inflammatory myocarditis may be crucial in order to determine prognosis. Such patients often present with rapidly progressing heart failure within weeks or months. Giant cell myocarditis may be particularly important to identify, as this carries a significantly worse prognosis and increased likelihood of requiring left ventricular assist device therapy or transplant.22 EMB can be an important tool to differentiate potential patients requiring transplantation or left ventricular assist device placement from those who may recover spontaneously.
The benefit of immunosuppressive treatment based on EMB findings is not entirely clear. Expert opinion and case series suggest immunosuppressive therapy may be of benefit in inflammatory myocarditis.23 The first major randomised trial (The Myocarditis Treatment Trial), published in 1995, failed to demonstrate mortality benefit from immunosuppression.24 Further randomised trials looking at virus-negative myocarditis cohorts were able to demonstrate improvements in left ventricular ejection fraction (LVEF) and decreased left ventricular end diastolic diameter (LVEDD).25,26 However, meta-analysis has not been able to detect significant reduction in mortality or need for transplantation with immunosuppressive therapies over usual care.27 Further registries and larger clinical trials may yet elucidate the best treatment for these patients.
Other uses of EMB
Amyloidosis may be diagnosed histologically on EMB. Despite this, the clinical presentation in combination with CMR and non-cardiac biopsy (i.e. rectal or nerve biopsy) are usually sufficient to make a diagnosis.28,29 Other rare conditions, such as eosinophilic myocarditis and cardiac sarcoidosis, can also be confirmed by EMB, but CMR and positron emission tomography–computed tomography (PET-CT) may be equally effective non-invasive diagnostic tools.30,31
Limitations of EMB
Diagnostic yield from EMB remains a challenge, often due to the location of the biopsy site.32 Routine right intraventricular septum sampling frequently misses specific areas of myocardial inflammation or fibrosis. This may not be surprising considering previous CMR studies have shown myocarditis more commonly affecting the left ventricle.33 Some centres perform biventricular and left ventricular biopsy, which appears to improve diagnostic yield.34,35 Identifying suitable biopsy sites using magnetic resonance imaging (MRI) and electrophysiology mapping have both been piloted, but are not largely available or standard practice.36,37
Use of EMB also relies on the availability of experienced pathologists trained in biopsy interpretation. The Dallas Criteria, proposed in 1986, are the agreed pathological criteria used both in trials and clinical practice to define myocarditis.38 However, the criteria do not take into account newer biopsy analysis techniques and there is significant inter-observer variability, even between expert pathologists.39,40
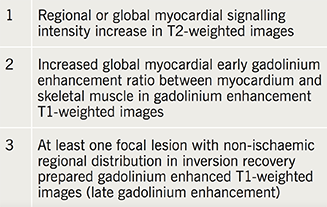
CMR versus EMB?
Clinical use of CMR as a non-invasive tool to diagnose myocardial diseases has risen with increasing availability. Lake Louise Criteria provide a pooled diagnostic accuracy of 78% with a sensitivity of 67% and specificity of 91% for diagnosis of myocarditis on CMR (table 3).41 However, more recent studies using T1- and T2-weighted mapping have shown further improvement in diagnostic performance.42 While EMB can be seen as a ‘gold-standard’ diagnostic test, CMR often provides a more immediately available investigation to the physician. Currently, no randomised trials have been performed comparing outcomes of non-invasive and invasive strategies for diagnosis of myocarditis. In practice, both strategies may be seen as complementary, rather than exclusive, and a diagnostic approach is often based on local resources.
Recent guidance and expert opinion on EMB
The European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Diseases produced a scientific statement regarding investigation and management of myocarditis in 2013.14 This group recommended, “all patients with clinically suspected myocarditis should be considered for selective coronary angiography and EMB”. They made this recommendation based on the advantages in newer diagnostic techniques available, particularly in viral myocarditis. The working group also proposed using EMB as a monitoring tool in those treated with immunosuppression for myocarditis. Equally, the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology have suggested EMB remains a helpful tool in diagnosis of myocardial diseases, and should still be included in diagnostic investigation.43
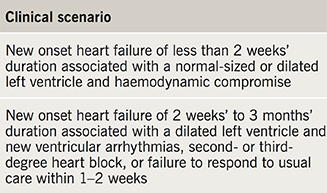
However, in 2007, an expert panel from the American Heart Association (AHA), the American College of Cardiology (ACC), and the ESC released a joint statement regarding EMB use in management of cardiovascular disease.44 This statement was meant to provide a more pragmatic and clinically relevant guideline for clinicians and has been widely endorsed. The authors outline 14 clinical scenarios where it was felt EMB might clarify a diagnostic strategy or affect clinical management. Of these, only two particular scenarios were suggested as class IB recommendation for use of EMB (table 4).
In these clinical scenarios, a histological diagnosis from biopsy may provide prognostic information or inform the physician as to which treatment strategy to take. In the same vein, the 2016 ESC guidelines for diagnosis and treatment of heart failure suggested that EMB might be considered in rapidly progressive heart failure when “there is a probability of a specific diagnosis which can only be confirmed in myocardial samples and specific therapy is available and effective” (IIbC).45 However, these do not represent common scenarios encountered in everyday clinical practice. As such, it is easier to understand the paucity of EMB service provision outside specialist centres. Crucially, there remains no clear answer as to whether an invasive or non-invasive strategy is superior in patients outside of transplant monitoring.
Conclusions
While EMB remains a useful diagnostic tool in specific scenarios, a broader application of this safe but invasive procedure is not agreed. Currently, in England, a limited number of NHS trusts perform EMB and the performance of EMB is rarely for investigation of myocarditis. EMB remains the gold-standard investigation for transplant monitoring, but there is uncertainty that an invasive diagnostic strategy is superior to non-invasive strategy in investigation of myocarditis or cardiomyopathy. There is a clinical need for a head-to-head randomised trial to clarify whether the newer diagnostic techniques involved in EMB can improve outcomes in these conditions over usual care or non-invasive cardiac imaging. Until this is ascertained, routine use of EMB will likely remain outside mainstream practice in England.
Key messages
- Endomyocardial biopsy (EMB) is an invasive procedure used to acquire a histological diagnosis in myocardial diseases
- EMB is not routinely performed in myocarditis in England. Use in monitoring of cardiac transplant patients is confined to tertiary centres
- Despite recent technological advances, there remain limitations and controversy regarding use of EMB
- Benefits of immunosuppression and antiviral treatments in myocarditis remain largely unproven
Funding
None.
Conflict of interest
None declared.
References
1. Sakakibara S, Konno S. Endomyocardial biopsy. Jpn Heart J 1962;3:537–43. https://doi.org/10.1536/ihj.3.537
2. Caves PK, Stinson EB, Graham AF, Billingham ME, Grehl TM, Shumway NE. Percutaneous transvenous endomyocardial biopsy. JAMA 1973;225:288–91. https://doi.org/10.1001/jama.1973.03220300044010
3. Richardson PJ. King’s endomyocardial bioptome. Lancet 1974;1:660–1. https://doi.org/10.1016/S0140-6736(74)93204-8
4. Blomstrom-Lundqvist C, Noor AM, Eskilsson J, Persson S. Safety of transvenous right ventricular endomyocardial biopsy guided by two-dimensional echocardiography. Clin Cardiol 1993;16:487–92. https://doi.org/10.1002/clc.4960160606
5. Cooper LT. The role of left ventricular biopsy in the management of heart disease. Circulation 2013;128:1492–4. https://doi.org/10.1161/CIRCULATIONAHA.113.005395
6. Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43–7. https://doi.org/10.1016/0735-1097(92)90049-S
7. Holzmann M, Nicko A, Kuhl U et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722–8. https://doi.org/10.1161/CIRCULATIONAHA.107.743427
8. From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc 2011;86:1095–102. https://doi.org/10.4065/mcp.2011.0296
9. Costanzo MR, Dipchand A, Starling R et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914–56. https://doi.org/10.1016/j.healun.2010.05.034
10. Orrego CM, Cordero-Reyes AM, Estep JD, Loebe M, Torre-Amione G. Usefulness of routine surveillance endomyocardial biopsy 6 months after heart transplantation. J Heart Lung Transplant 2012;31:845. https://doi.org/10.1016/j.healun.2012.03.015
11. Shah KB, Flattery MP, Smallfield MC et al. Surveillance endomyocardial biopsy in the modern era produces low diagnostic yield for cardiac allograft rejection. Transplantation 2015;99:e75–e80. https://doi.org/10.1097/TP.0000000000000615
12. Butler CR, Savu A, Bakal JA et al. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Heart Lung Transplant 2015;34:643–50. https://doi.org/10.1016/j.healun.2014.12.020
13. Kobashigawa J, Patel J, Azarbal B et al. Randomized pilot trial of gene expression profiling versus heart biopsy in the first year after heart transplant: early invasive monitoring attenuation through gene expression trial (EIMAGE). Circ Heart Fail 2015;8:557–64. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001658
14. Caforio AL, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–48. https://doi.org/10.1093/eurheartj/eht210
15. Kühl U, Pauschinger M, Noutsias M et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005;111:887–93. https://doi.org/10.1161/01.CIR.0000155616.07901.35
16. Why HJ, Meany BT, Richardson PJ et al. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation 1994;89:2582–9. https://doi.org/10.1161/01.CIR.89.6.2582
17. Maekawa Y, Ouzounian M, Opavsky MA, Liu PP. Connecting the missing link between dilated cardiomyopathy and viral myocarditis virus, cytoskeleton, and innate immunity. Circulation 2007;115:5–8. https://doi.org/10.1161/CIRCULATIONAHA.106.670554
18. Matsumori A, Tomioka N, Kawai C. Protective effect of recombinant alpha interferon on coxsackievirus B3 myocarditis in mice. Am Heart J 1988;115:1229–32. https://doi.org/10.1016/0002-8703(88)90013-0
19. Kühl U, Lassner D, von Schlippenbach J, Poller W, Schultheiss HP. Interferon-beta improves survival in Enterovirus-associated cardiomyopathy. J Am Coll Cardiol 2012;60:1295–6. https://doi.org/10.1016/j.jacc.2012.06.026
20. Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev 2013;(10):CD004471. https://doi.org/10.1002/14651858.cd004471.pub3
21. Robinson J, Hartling L, Vandermeer B, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev 2005;(1):CD004370. https://doi.org/10.1002/14651858.cd004370.pub2
22. Cooper Jr LT, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis – natural history and treatment. N Engl J Med 1997;336:1860–6. https://doi.org/10.1056/NEJM199706263362603
23. Cooper LT, Hare JM, Tazelaar HD et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 2008;102:1535–9. https://doi.org/10.1016/j.amjcard.2008.07.041
24. Mason JW, O’Connell JB, Herskowitz A et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269–75. https://doi.org/10.1056/NEJM199508033330501
25. Wojnicz R, Nowalany-Kozielska E, Wojciechowska C et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy. Circulation 2001;104:39–45. https://doi.org/10.1161/01.CIR.104.1.39
26. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009;30:1995–2002. https://doi.org/10.1093/eurheartj/ehp249
27. Lu C, Qin F, Yan Y, Liu T, Li J, Chen H. Immunosuppressive treatment for myocarditis: a meta-analysis of randomized controlled trials. J Cardiovasc Med 2016;17:631–7. https://doi.org/10.2459/JCM.0000000000000134
28. Syed IS, Glockner JF, Feng D et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 2010;3:155–64. https://doi.org/10.1016/j.jcmg.2009.09.023
29. Fine NM, Arruda-Olson AM, Dispenzieri A et al. Yield of noncardiac biopsy for the diagnosis of transthyretin cardiac amyloidosis. Am J Cardiol 2014;113:1723–7. https://doi.org/10.1016/j.amjcard.2014.02.030
30. Kuchynka P, Palecek T, Masek M et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int 2016;2016:2829583. https://doi.org/10.1155/2016/2829583
31. Yoshida A, Ishibashi-Ueda H, Yamada N et al. Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure. Eur J Heart Fail 2013;15:166–75. https://doi.org/10.1093/eurjhf/hfs206
32. Chow LH, Radio SJ, Sears TD, Mcmanus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol 1989;14:915–20. https://doi.org/10.1016/0735-1097(89)90465-8
33. Mahrholdt H, Goedecke C, Wagner A et al. Cardiovascular magnetic resonance assessment of human myocarditis. Circulation 2004;109:1250–8. https://doi.org/10.1161/01.CIR.0000118493.13323.81
34. Yilmaz A, Kindermann I, Kindermann M et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010;122:900–9. https://doi.org/10.1161/CIRCULATIONAHA.109.924167
35. Stiermaier T, Föhrenbach F, Klingel K et al. Biventricular endomyocardial biopsy in patients with suspected myocarditis: feasibility, complication rate and additional diagnostic value. Int J Cardiol 2017;230:364–70. https://doi.org/10.1016/j.ijcard.2016.12.103
36. Rogers T, Ratnayaka K, Karmarkar P et al. Real-time magnetic resonance imaging guidance improves the diagnostic yield of endomyocardial biopsy. JACC Basic Transl Sci 2016;1:376–83. https://doi.org/10.1016/j.jacbts.2016.05.007
37. Casella M, Pizzamiglio F, Russo AD et al. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ Arrhythm Electrophysiol 2015;8:625–32. https://doi.org/10.1161/circep.114.002216
38. Aretz HT, Billingham ME, Edwards WD et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3–14.
39. Shanes JG, Ghali J, Billingham ME et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 1987;75:401–05. https://doi.org/10.1161/01.CIR.75.2.401
40. Baughman KL. Diagnosis of myocarditis. Circulation 2006;113:593–5. https://doi.org/10.1161/CIRCULATIONAHA.105.589663
41. Friedrich MG, Sechtem U, Schulz-Menger J et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475–87. https://doi.org/10.1016/j.jacc.2009.02.007
42. Lurz P, Luecke C, Eitel I et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol 2016;67:1800–11. https://doi.org/10.1016/j.jacc.2016.02.013
43. Thiene G, Bruneval P, Veinot J, Leone O. Diagnostic use of the endomyocardial biopsy: a consensus statement. Virchows Archiv 2013;463:1–5. https://doi.org/10.1007/s00428-013-1430-4
44. Cooper LT, Baughman KL, Feldman AM et al. The role of endomyocardial biopsy in the management of cardiovascular disease. Eur Heart J 2007;28:3076–93. https://doi.org/10.1093/eurheartj/ehm456
45. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128
