Atrial fibrillation (AF) is known to increase stroke risk and can be stratified clinically by the CHA2DS2-VASc scoring system, which then informs recommendations for long-term anticoagulation. Susceptibility to thromboembolism is also increased around the time of catheter ablation of AF. Mechanistically, this is accounted for by endothelial injury, hypercoagulability due to contact of blood with foreign surfaces and altered blood flow after conversion to normal sinus rhythm (figure 1).1 The risk of stroke persists post-ablation, even in patients with low CHA2DS2-VASc scores, as the atria may remain stunned for several weeks post-ablation, and the endothelium takes time to heal. This phenomenon forms the rationale for guidelines currently recommending anticoagulation for two to three months post-ablation.2
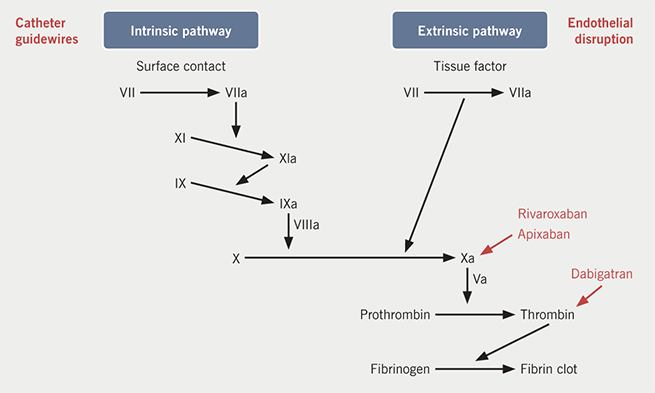
So how do we mitigate the thromboembolic risk during catheter ablation? Intravenous (IV) heparin is used with an activated clotting time (ACT) target of >300 s during the procedure and the use of saline-irrigated catheters seems to reduce risk further by decreasing the incidence of emboli from the catheter tip.3 Guidelines currently recommend oral anticoagulation three to four weeks before ablation,2,4 but there remains some debate about the management of oral anticoagulation at the time of the procedure. Two management options exist; the first is bridging, where the anticoagulant is interrupted pre-procedure and subcutaneous heparin replaces it, with the dose missed on the day of the procedure. This exposes the patient to the risk of embolus during the immediate post-procedure period because the patient has no anticoagulation between the time the IV heparin wears off and the next dose of subcutaneous heparin is given. The alternative is to continue the anticoagulant uninterrupted. This means that patients are always anticoagulated, but does increase the potential for bleeding complications during the procedure.1 This rationale has been tested in a retrospective study by Di Biase et al. in 2010, which showed uninterrupted warfarin reduced stroke and major bleeding events.5
This was followed by the COMPARE (Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation Patients Undergoing Catheter Ablation) trial,6 a prospective randomised trial showing reduced stroke risk and minor bleeding with an uninterrupted warfarin strategy, when compared with low molecular weight heparin (LMWH) bridging. The major bleeding rates were similar. Although carried out in patients having pacemaker implants, a higher risk of pocket haematoma with heparin or LMWH bridging compared with uninterrupted warfarin was seen in the BRUISE-CONTROL trial (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial),7 giving further weight to the argument that bridging puts patients at an increased risk of bleeding peri-ablation. Furthermore, performing ablation with uninterrupted warfarin has been shown to be associated with fewer bleeding complications even when not using intra-cardiac ultrasound to guide the transeptal puncture.8
Non-vitamin K antagonist oral anticoagulants (NOACs) offer a more predictable anticoagulant effect than warfarin, and the convenience of not needing regular monitoring. The stable anticoagulant action can obviate the need for pre-ablation transoesophageal echo (TOE) to exclude left atrial appendage thrombus, often the consequence of subtherapeutic international normalised ratio (INR) values in the run up to ablation.9 Conversely, high INR values can also preclude AF ablation, and it is the practice at our institute to use a cut-off INR of 3.5. This has led to their increasing prevalence in the mitigation of stroke risk in AF patients.
While the more predictable pharmacodynamics of these drugs offers a seductive rationale for their use, this was initially limited because of concerns about safety and the ability to reverse them if a catastrophic bleed occurred during the procedure. This concern seems to be difficult to justify when one acknowledges that in randomised trials the frequency and severity of bleeds appear to be similar to those for aspirin and NOACs,10 and yet aspirin has no specific reversal agent and is rarely interrupted for cardiac procedures.
While they do influence clotting screens, with a raised prothrombin time (PT) being seen with factor Xa inhibitors,11 and raised activated partial thromboplastin time (aPTT) and thrombin time (TT) with dabigatran,12,13 these are not used to monitor the magnitude of the anticoagulant effect. Despite the lack of monitoring being, for the most part, beneficial, the inability to monitor anticoagulant effect could also be problematic in certain clinical scenarios, such as during major bleeds. Other problems exist, such as the need for higher intra-operative dosing of heparin to achieve desired ACT.14,15
Given the initial lack of clinical data on peri-procedural use of NOACs, one strategy employed was transitioning from NOAC to warfarin in the run up to ablation. However, given a post-hoc analysis of the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial showed a higher stroke rate in patients transitioned to warfarin from NOAC,16 this may be ill-advised. Especially given that recent evidence has been reassuring for NOAC use.
The RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to warfarIn in pulmonaRy Vein Ablation: Assessment of an Uninterrupted periproCedUral antIcoagulation sTrategy) trial is a randomised-controlled, open-label study comparing uninterrupted warfarin to uninterrupted dabigatran. The results showed a significant reduction in bleeding events in the dabigatran arm, with one thromboembolic event occurring in the warfarin arm. The lower bleeding rate is difficult to account for physiologically, and may be due to the unblinded nature of the trial.17 The results are nonetheless reassuring.
A further important take-home message from the trial is the way major bleeds were managed. There is often concern among patients regarding the lack of an antidote for NOACs, this concern persists and is shared by many clinicians. In the RE-CIRCUIT trial, none of the major bleeding events required the use of an antidote (which was available during the trial).17 This may provide further reassurance of the risks associated with NOAC use.
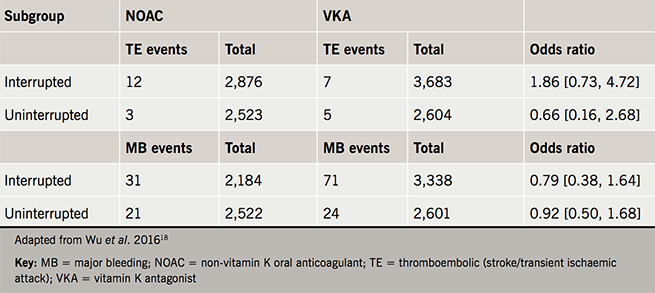
Another randomised-controlled trial, this time analysing rivaroxaban versus warfarin in an uninterrupted strategy, showed no significant difference in bleeding events with one thromboembolic event occurring in the warfarin arm of the trial, although the numbers studied were low, making conclusions more difficult.15 In a meta-analysis comparing uninterrupted warfarin therapy with NOACs (dabigatran, rivaroxaban and apixaban) it was found that minor bleeding was significantly lower in the NOAC cohorts and no difference was seen in stroke/transient ischaemic attack (TIA) and major bleeding between the groups (table 1).18
Conclusion
Recent evidence has shown that an uninterrupted anticoagulation strategy is preferable and reduces stroke while minimising bleeding risk. The availability of NOACs initially caused concern about an increased bleeding risk and lack of antidote, but this has not been borne out in studies. For the purposes of catheter ablation of AF, NOACs are safe to use and the data suggest they should be used in an uninterrupted manner. Whether this approach extends to other procedures in patients at high risk of stroke is yet to be seen, but unlike catheter ablation, there are few other cardiac procedures that result in an increased risk of stroke because there is no instrumentation or endothelial damage of left-sided structures with areas of slow blood flow as occurs in the left atrium.
Conflict of interest
None declared.
References
1. Weitz JI, Healey JS, Skanes AC, Verma A. Periprocedural management of new oral anticoagulants in patients undergoing atrial fibrillation ablation. Circulation 2014;129:1688–94. https://doi.org/10.1161/CIRCULATIONAHA.113.005376
2. Calkins H, Kuck KH, Cappato R et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol 2012;33:171–257. https://doi.org/10.1007/s10840-012-9672-7
3. Cappato R, Calkins H, Chen S-A et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. https://doi.org/10.1161/CIRCEP.109.859116
4. Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. https://doi.org/10.1093/eurheartj/ehw210
5. Di Biase L, Burkhardt JD, Mohanty P et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010;121:2550–6. https://doi.org/10.1161/CIRCULATIONAHA.109.921320
6. Di Biase L, Burkhardt JD, Santangeli P et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patient. Circulation 2014;129:2638. https://doi.org/10.1161/CIRCULATIONAHA.113.006426
7. Birnie DH, Healey JS, Wells GA et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–93. https://doi.org/10.1056/NEJMoa1302946
8. Page SP, Siddiqui MS, Finlay M et al. Catheter ablation for atrial fibrillation on uninterrupted warfarin: can it be done without echo guidance? J Cardiovasc Electrophysiol 2011;22:265–70. https://doi.org/10.1111/j.1540-8167.2010.01910.x
9. Maan A, Heist EK, Ruskin JN, Mansour M. Practical issues in the management of novel oral anticoagulants – cardioversion and ablation. J Thorac Dis 2015;7:115–31. https://doi.org/10.3978/j.issn.2072-1439.2014.11.35
10. Connolly S, Eikelboom J, Joyner C et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. https://doi.org/10.1056/NEJMoa1007432
11. Douxfils J, Mullier F, Loosen C et al. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 2012;130:956–66. https://doi.org/10.1016/j.thromres.2012.09.004
12. Lindahl TL, Baghaei F, Blixter IF et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost 2011;105:371–8. https://doi.org/10.1160/TH10-06-0342
13. van Ryn J, Stangier J, Haertter S et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–27. https://doi.org/10.1160/TH09-11-0758
14. Snipelisky D, Ray JC, Ung R, Duart M, Kauffman C, Kusumoto F. A comparison of bleeding complications between warfarin, dabigatran, and rivaroxaban in patients undergoing cryoballoon ablation. J Interv Card Electrophysiol 2014;41:231–6. https://doi.org/10.1007/s10840-014-9948-1
15. Cappato R, Marchlinski FE, Hohnloser SH et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–11. https://doi.org/10.1093/eurheartj/ehv177
16. Patel MR, Hellkamp AS, Lokhnygina Y et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013;61:651–8. https://doi.org/10.1016/j.jacc.2012.09.057
17. Calkins H, Willems S, Gerstenfeld EP et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. https://doi.org/10.1056/NEJMoa1701005
18. Wu S, Yang YM, Zhu J et al. Meta-analysis of efficacy and safety of new oral anticoagulants compared with uninterrupted vitamin K antagonists in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol 2016;117:926–34. https://doi.org/10.1016/j.amjcard.2015.12.027
